New and known free-living nematode species (Nematoda: Chromadorea) from offshore tsunami monitoring buoys in the Southwest Pacific Ocean
- PMID: 40755792
- PMCID: PMC12317693
- DOI: 10.7717/peerj.19789
New and known free-living nematode species (Nematoda: Chromadorea) from offshore tsunami monitoring buoys in the Southwest Pacific Ocean
Abstract
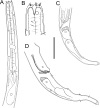
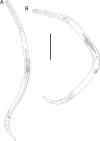
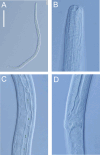
Deep-ocean Assessment and Reporting of Tsunami (DART) buoys are deployed across the Southwest Pacific and provide substrates for biofouling communities. Two new free-living nematode species, Atrochromadora tereroa sp. nov. and Euchromadora rebeccae sp. nov. (family Chromadoridae), and one known species, Halomonhystera refringens (Bresslau & Schuurmans Stekhoven, 1933) comb. nov. (family Monhysteridae), are described from buoys deployed off Raoul Island in the Kermadec/Rangitāhua region and off New Zealand's East Cape. Thalassomonhystera refringens (Bresslau & Schuurmans Stekhoven, 1933) Jacobs, 1987 and T. anoxybiotica (Jensen, 1986) Jacobs, 1987 are transferred to Halomonhystera based on the presence of precloacal and caudal papillae in males. In addition, Halomohystera zhangi Li, Huang & Huang, 2024 is synonymised with Halomonhystera refringens. Updated keys to Atrochromadora, Euchromadora and Halomonhystera species are provided. The presence of nematodes on buoys located more than 100 km from the nearest landmass and in deep waters (>3,500 m water depth) shows that some nematode species are capable long-distance dispersal to colonise new substrates. Such dispersal by Atrochromadora, Euchromadora and Halomonhystera species likely occurs via drifting macroalgal fragments.
Keywords: Chromadorida; Epiphytic; Monhysterida; Nematoda; New species.
©2025 Leduc.
Conflict of interest statement
The authors declare there are no competing interests.
Figures









Similar articles
-
Swedish Plectida (Nematoda). Part 4. The genus Leptolaimus de Man, 1876.Zootaxa. 2013 Nov 25;3739:1-99. doi: 10.11646/zootaxa.3739.1.1. Zootaxa. 2013. PMID: 25112880
-
NEOLEBOURIA MULLINEAUXAE N. SP. (TREMATODA: DIGENEA) AND ANOTHER OPECOELID FROM DEEP-SEA HYDROTHERMAL VENT FIELDS OFF CENTRAL AMERICA AND PAPUA NEW GUINEA, WITH SPECIES KEYS AND A COMPARISON TO MESOBATHYLEBOURIA.J Parasitol. 2025 Jul 1;111(4):451-478. doi: 10.1645/24-113. J Parasitol. 2025. PMID: 40659347
-
Two new species and a new record of Comesomatidae (Nematoda, Araeolaimida) from the Southern Ocean.Zookeys. 2025 Jul 8;1244:121-145. doi: 10.3897/zookeys.1244.135491. eCollection 2025. Zookeys. 2025. PMID: 40672780 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Telehealth interventions: remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2021 Jul 20;7(7):CD013196. doi: 10.1002/14651858.CD013196.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693988 Free PMC article.
References
-
- Allgén CA. Weitere Beiträge zur Kenntnis der marinen Nematodenfauna der Campbellinsel. Nyt Magazin for Naturvidenskaberne. 1932;70:97–198.
-
- Apolonio Silva de Oliveira D, Decraemer D, Moens T, Dos Santos GAP, Derycke S. Low genetic but high morphological variation over more than 1,000 km coastline refutes omnipresence of cryptic diversity in marine nematodes. BMC Evolutionary Biology. 2017;17:71. doi: 10.1186/s12862-017-0908-0. - DOI - PMC - PubMed
-
- Boeckner MJ, Sharma J, Proctor HC. Revisiting the meiofauna paradox: dispersal and colonization of nematodes and other meiofaunal organisms in low- and high-energy environments. Hydrobiologia. 2009;62:91–106. doi: 10.1007/s10750-008-9669-5. - DOI
-
- Bresslau E, Schuurmans Stekhoven Jr JH. Marine freilebende Nematoda aus der Nordsee. Musée d’Histoire Naturelle de Belgique; Bruxelles: 1940. p. 74.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

