Strategic incorporation of metal ions in bone regenerative scaffolds: multifunctional platforms for advancing osteogenesis
- PMID: 40755870
- PMCID: PMC12317318
- DOI: 10.1093/rb/rbaf068
Strategic incorporation of metal ions in bone regenerative scaffolds: multifunctional platforms for advancing osteogenesis
Abstract
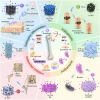
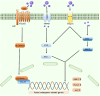
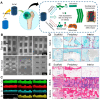
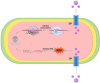
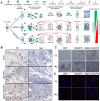
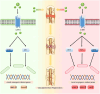
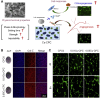
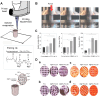
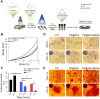
Bone serves as a critical structural framework, enabling movement and protecting internal organs. Consequently, maintaining skeletal health is a pivotal objective in bone tissue engineering. Bioactive metal ions, such as magnesium, strontium, zinc and copper, play essential roles in bone metabolism by participating in key physiological processes that sustain bone health and support regeneration. Recent studies indicate that these ions enhance the physicochemical properties and biological performance of bone tissue engineering materials, thereby facilitating osseointegration through diverse mechanisms. Specifically, magnesium promotes osteogenic differentiation; strontium inhibits osteoclast activity; zinc exhibits antibacterial properties; and copper facilitates vascularization for osteogenesis. Therefore, incorporating bioactive metal ions has emerged as a prevalent strategy in bone tissue engineering to address orthopedic disorders. This review systematically summarizes the roles of magnesium, strontium, zinc and copper in bone repair and regeneration. It provides an in-depth analysis of engineered materials incorporating these ions, with a focus on their applications and modifications across various material types. Furthermore, we explore the synergistic effects of combining these metal ions in bone tissue engineering, emphasizing their enhanced biological properties. By synthesizing recent research findings, this review aims to provide new insights and potential breakthroughs in leveraging bioactive metal ions for advancing treatments of orthopedic diseases.
Keywords: bone regeneration; bone tissue engineering materials; metal ions; osteopororsis.
© The Author(s) 2025. Published by Oxford University Press.
Figures

Similar articles
-
Extracellular vesicles derived from Schwann cells to enhance bone and dental tissue regeneration: a literature review.J Nanobiotechnology. 2025 Jul 11;23(1):502. doi: 10.1186/s12951-025-03585-7. J Nanobiotechnology. 2025. PMID: 40646600 Free PMC article. Review.
-
Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors.J Control Release. 2014 Nov 10;193:282-95. doi: 10.1016/j.jconrel.2014.04.026. Epub 2014 Apr 26. J Control Release. 2014. PMID: 24780264
-
Property-tailoring chemical modifications of hyaluronic acid for regenerative medicine applications.Acta Biomater. 2025 Jul 1;201:75-100. doi: 10.1016/j.actbio.2025.06.014. Epub 2025 Jun 7. Acta Biomater. 2025. PMID: 40490241 Review.
-
An Exploration of the Role of Osteoclast Lineage Cells in Bone Tissue Engineering.Tissue Eng Part B Rev. 2025 Jun;31(3):248-265. doi: 10.1089/ten.TEB.2024.0126. Epub 2024 Aug 19. Tissue Eng Part B Rev. 2025. PMID: 39041616 Review.
-
Bio-response of copper-magnesium co-substituted mesoporous bioactive glass for bone tissue regeneration.J Mater Chem B. 2024 Feb 14;12(7):1875-1891. doi: 10.1039/d3tb01568h. J Mater Chem B. 2024. PMID: 38293829
References
-
- Kraft MD. Phosphorus and calcium: a review for the adult nutrition support clinician. Nutr Clin Pract 2015;30:21–33. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

