Desensitization in HLA-incompatible kidney transplant recipients: current strategies and emerging perspectives
- PMID: 40755969
- PMCID: PMC12315108
- DOI: 10.1093/ckj/sfaf219
Desensitization in HLA-incompatible kidney transplant recipients: current strategies and emerging perspectives
Abstract
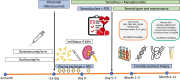
Despite development of kidney paired donation programs and prioritization in kidney allocation schemes, transplantation rates are still low and waiting times remain prolonged for highly sensitized kidney transplant recipients with broad human leukocyte antigen antibody reactivity. Desensitization confers an invaluable option improving access to kidney transplantation for sensitized patients who could not benefit from kidney paired donation programs and kidney allocation schemes. Conventional desensitization strategies use intravenous immunoglobulin combined with either plasmapheresis or monoclonal anti-CD20 antibodies. Imlifidase, IL-6 targeting agents, plasma cell-directed therapies, complement inhibitors, chimeric antigen receptor T-cell therapies, and B cell-activating factor inhibitors are emerging new options in the hope of enhancing and sustaining the efficacy of desensitization to improve allograft longevity. In this review, we discuss the rationale and outcome of desensitization with various strategies alone or in combination. Our aim is also to provide some insight for decision when pursuing desensitization might be successful or futile in sensitized patients.
Keywords: CAR T-cell therapies; desensitization; kidney transplantation; plasma cell-directed therapies; plasmapheresis.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
None of the authors have anything to disclose.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

