Safety Profile of Intravenous Ferulic Acid Nanoparticles: Acute Toxicity and Neurological Effects in Sprague-Dawley Rats
- PMID: 40756009
- PMCID: PMC12315901
- DOI: 10.2147/NSA.S500407
Safety Profile of Intravenous Ferulic Acid Nanoparticles: Acute Toxicity and Neurological Effects in Sprague-Dawley Rats
Abstract
Background: Ferulic acid (FA) exhibits therapeutic potential for various disorders, but its clinical application is hindered by poor bioavailability and solubility. This study aimed to develop and evaluate FA-loaded lipid nanoparticles (FA-LNPs) as a safe and efficient drug delivery system.
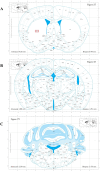
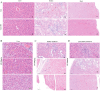
Methods: FA-LNPs were prepared via an optimized active loading method. The Morris water maze test was conducted to evaluate FA efficacy against LPS-induced cognitive impairment in rats. Comprehensive neurotoxicity assessment was performed in three brain regions (striatum, hippocampus, and cerebellum-brain stem) using multiple staining techniques (LFB, GFAP, IBA-1, and Fluoro-Jade) to evaluate myelin integrity, glial activation, and neuronal degeneration. Acute toxicity, pharmacokinetics, and network pharmacology analysis were conducted to assess safety profiles and potential mechanisms.
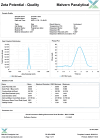
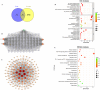
Results: FA-LNPs were successfully prepared using an optimized active loading method, achieving high drug loading (≥4 mg/mL), superior encapsulation efficiency (EE%) ≥80%, and uniform particle size distribution (<200 nm, PDI=0.053), zeta potential of +5.97 mV (Quality Factor = 1.701), excellent storage stability over two weeks, and was scaled up for batch production. The Morris water maze test revealed an effective FA concentration of 50 mg/kg, with FA-LNPs achieving 46.5 mg/kg through active loading method. Toxicological studies demonstrated favorable safety profiles. Pharmacokinetic analysis showed a prolonged elimination half-life (12.8 ± 1.88 hours) and moderate systemic clearance (0.535 ± 0.0851 L/h/kg). Short-term administration did not elicit significant neuroprotection. Network pharmacology analysis identified 141 potential therapeutic targets and five key proteins (EGFR, ESR1, PTGS2, CTNNB1, and STAT3), with molecular docking confirming favorable binding energies (-7.6 to -5.2 kcal/mol).
Conclusion: FA-LNPs enhanced FA's bioavailability without apparent systemic toxicity or neurotoxicity. While safe for short-term use, longer treatment durations may be necessary to observe potential neuroprotective benefits and toxicity. This study provides a foundation for further investigation of FA-LNPs as a promising drug delivery system for neurological disorders.
Keywords: Alzheimer’s disease; atherosclerosis; drug delivery; ferulic acid; liposome nanoparticles; network pharmacology; pharmacokinetics; toxicology.
© 2025 Huang et al.
Conflict of interest statement
The authors report no conflicts of interest.
Figures













References
-
- Ou L, Kong LY, Zhang XM, Niwa M. Oxidation of ferulic acid by momordica charantia peroxidase and related anti-inflammation activity changes. Biol Pharm Bull. 2003;26(11):1511–1516. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

