Transition-State Stabilization by Secondary Orbital Interactions between Fluoroalkyl Ligands and Palladium During Reductive Elimination from Palladium(aryl)(fluoroalkyl) Complexes
- PMID: 40756021
- PMCID: PMC12316061
- DOI: 10.1021/acscatal.3c02648
Transition-State Stabilization by Secondary Orbital Interactions between Fluoroalkyl Ligands and Palladium During Reductive Elimination from Palladium(aryl)(fluoroalkyl) Complexes
Abstract
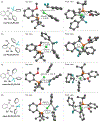
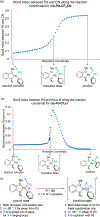
Palladium-catalyzed fluoroalkylations of aryl halides are valuable reactions for the synthesis of fluorinated, biologically active molecules. Reductive elimination from an intermediate Pd(aryl) (fluoroalkyl) complex is the step that forms the C(aryl)-C(fluoroalkyl) bond, and this step typically requires higher temperatures and proceeds with slower rates than the reductive elimination of nonfluorinated alkylarenes from the analogous Pd(aryl) (alkyl) complexes. The experimental rates of this step correlate poorly with common parameters, such as the steric property or the electron-withdrawing ability of the fluoroalkyl ligand, making the prediction of rates and the rational design of Pd-catalyzed fluoroalkylations difficult. Therefore, a systematic study of the features of fluoroalkyl ligands that affect the barrier to this key step, including steric properties, electron-withdrawing properties, and secondary interactions, is necessary for the future development of fluoroalkylation reactions that occur under milder conditions and that tolerate additional types of fluoroalkyl reagents. We report computational studies of the effect of the fluoroalkyl ligand on the barriers to reductive elimination from complexes ( , , etc.) containing the bidentate ligand di-tert-butyl(2-methoxyphenyl)phosphine (L). The computed Gibbs free-energy barriers to reductive elimination from these complexes suggest that fluoroalkylarenes should form quickly at room temperature for the fluoroalkyl ligands we studied, excluding , , , , , , or . Analyses of the transition-state structures by natural bond orbital (NBO) and independent gradient model (IGMH) approaches reveal that orbital interactions between the Pd center and a hydrogen atom or -acid bonded to the -carbon atom of the ligand stabilize the lowest-energy transition states of complexes. Comparisons between conformers of transition-state structures suggest that the magnitude of such stabilizations is 4.7-9.9kcal/mol. In the absence of these secondary orbital interactions, a more electron-withdrawing fluoroalkyl ligand leads to a higher barrier to reductive elimination than a less electron-withdrawing fluoroalkyl ligand. Computations on the reductive elimination from complexes containing para-substituted aryl groups on palladium reveal that the barriers to reductive elimination from complexes containing more electron-rich aryl ligands tend to be lower than those to reductive elimination from complexes containing less electron-rich aryl ligands when the fluoroalkyl ligands of these complexes can engage in secondary orbital interactions with the metal center. However, the computed barriers to reductive elimination do not depend on the electronic properties of the aryl ligand when the fluoroalkyl ligands do not engage in secondary orbital interactions with the metal center.
Keywords: DFT calculations; fluoroalkylation; palladium catalysis; reductive elimination; secondary orbital interactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
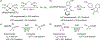







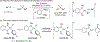
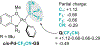
Similar articles
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Böhm H-J; Banner D; Bendels S; Kansy M; Kuhn B; Müller K; Obst-Sander U; Stahl M Fluorine in Medicinal Chemistry. ChemBioChem. 2004, 5, 637–643. - PubMed
-
- Dolbier WR Fluorine chemistry at the millennium. J. Fluorine Chem 2005, 126, 157–163.
-
- Gillis EP; Eastman KJ; Hill MD; Donnelly DJ; Meanwell NA Applications of Fluorine in Medicinal Chemistry. J. Med. Chem 2015, 58, 8315–8359. - PubMed
-
- Jeschke P The Unique Role of Fluorine in the Design of Active Ingredients for Modern Crop Protection. ChemBioChem. 2004, 5, 570–589. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
