Efficacy and safety of oral amantadine in Parkinson's disease with dyskinesia and motor fluctuations: a systematic review and meta-analysis of randomised controlled trials
- PMID: 40756069
- PMCID: PMC12314832
- DOI: 10.1136/bmjno-2025-001115
Efficacy and safety of oral amantadine in Parkinson's disease with dyskinesia and motor fluctuations: a systematic review and meta-analysis of randomised controlled trials
Abstract
Background: Oral amantadine is available in three formulations with distinct pharmacokinetics: immediate-release (IR), delayed-release/extended-release (DR/ER) and immediate-release/extended-release (IR/ER). While all formulations alleviate levodopa-induced dyskinesia, only DR/ER has shown efficacy for motor fluctuations. This meta-analysis evaluates the impact of amantadine formulations on motor complications in Parkinson's disease (PD).
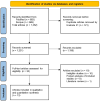
Methods: A systematic search of PubMed and Scopus (inception to February 2024) identified randomised controlled trials (RCTs) evaluating dyskinesia using various Dyskinesia Rating Scales (DRS) and Unified Parkinson's Disease Rating Scale (UPDRS) or Movement Disorder Society (MDS)-UPDRS part 4 subscores ((MDS-)UPDRS IV), motor fluctuations using 'OFF' time and safety through adverse events. Subgroup analysis assessed formulation-specific effects. The I² statistic determined the use of fixed-effects or random-effects models for efficacy outcomes. Dyskinesia was analysed using standardised mean difference (SMD), motor fluctuations with mean difference (MD) and adverse events with ORs via a fixed-effects Mantel-Haenszel model.
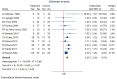
Results: Fourteen RCTs (13 articles) were included. Amantadine significantly reduced dyskinesia (DRS: SMD=-1.32, 95% CI (-1.78 to -0.86); (MDS-)UPDRS IV: SMD=-0.95, 95% CI (-1.33 to -0.58)), with similar effects across formulations. 'OFF' time decreased significantly (MD=-0.66, 95% CI (-0.93 to -0.40)), notably with IR (MD=-0.75, 95% CI (-1.41 to -0.10)) and DR/ER (MD=-0.96, 95% CI (-1.35 to -0.57)), but not IR/ER (MD=-0.23, 95% CI (-0.68 to 0.22)). Adverse events (OR=3.30, 95% CI (2.29 to 4.74)) included dry mouth, hallucinations, peripheral oedema, dizziness and constipation.
Conclusions: All amantadine formulations alleviated dyskinesia. Additionally, DR/ER improved motor fluctuations, while IR demonstrated benefits, although the evidence is limited by short study durations.
Prospero registration number: CRD42024513081.
Keywords: META-ANALYSIS; MOVEMENT DISORDERS; NEUROPHARMACOLOGY; PARKINSON'S DISEASE.
Copyright © Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
No, there are no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
