Decoding metabolic trade-offs in Halomonas elongata: Chemostat-based flux remodeling for industrial ectoine biosynthesis
- PMID: 40756072
- PMCID: PMC12318276
- DOI: 10.1016/j.synbio.2025.07.004
Decoding metabolic trade-offs in Halomonas elongata: Chemostat-based flux remodeling for industrial ectoine biosynthesis
Abstract
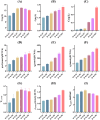
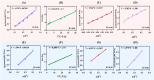
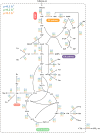
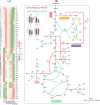
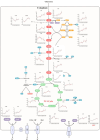
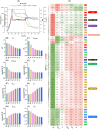
Halomonas elongata, a moderately halophilic γ-proteobacterium of industrial interest, serves as a microbial cell factory for ectoine-a high-value compatible solute extensively utilized in biopharmaceuticals and cosmetics. While its ectoine biosynthesis potential is well-documented, the systemic metabolic adaptations underlying osmoadaptation remain poorly characterized, limiting rational engineering strategies for optimized production. To address this gap, we employed chemostat cultivation coupled with multi-omics integration (physiological profiling, metabolomics, and metabolic flux analysis) to dissect salt-dependent metabolic network rewiring in the model strain DSM 2581T under moderate (6.0 % NaCl) and high salinity (13.0 % NaCl). Results demonstrated that, under moderate salt conditions, a specific growth rate (μ) of 0.20 h-1 significantly enhanced the ectoine-specific production rate (q p), intracellular ectoine content (p ectoine), and yield coefficient (Y p/s), concurrent with redirection of carbon flux toward the Entner-Doudoroff (ED) pathway and ectoine biosynthesis. Under high salt conditions, flux through both the ED pathway and ectoine biosynthesis was further upregulated, whereas fluxes through the pentose phosphate (PP) pathway, tricarboxylic acid (TCA) cycle, and CO2 generation were downregulated. Simultaneously, suppression of the flux from malate to pyruvate enhanced oxaloacetate synthesis, thereby increasing the supply of key precursors including glutamate, aspartate, and NADPH to fuel ectoine biosynthesis. Stepwise salt reduction experiments revealed bidirectional metabolic flexibility: elevated salinity prioritized carbon investment into ED-driven ectoine production, whereas hypo-osmotic conditions reactivate respiratory activity and the TCA cycle to fuel energy metabolism. These findings establish H. elongata as a paradigm of dynamic flux rewiring, where carbon economy is strategically reallocated between stress-protective solute biosynthesis and energy homeostasis. This study bridges the knowledge gap in understanding the physiological characteristics of H. elongata and provides a foundation for improving ectoine production and engineering strains through metabolic optimization.
Keywords: Chemostat culture; Ectoine biosynthesis; Halomonas elongata; Metabolic flux analysis; Salt adaptation.
© 2025 The Authors.
Figures
References
-
- Biswas J., Jana S.K., Mandal S. Biotechnological impacts of Halomonas: a promising cell factory for industrially relevant biomolecules. Biotechnol Genet Eng Rev. 2023;39(2):348–377. - PubMed
-
- Chen G.Q., Zhang X., Liu X., Huang W.R., Xie Z.W., Han J., Xu T., Mitra R., Zhou C., Zhang J., Chen T. Halomonas spp., as chassis for low-cost production of chemicals. Appl Microbiol Biotechnol. 2022;106(21):6977–6992. - PubMed
-
- Zhang Y.H., Xue C.M., Chen B.T., Ouyang P.F., Ling C. Comparing three emerging industrial cell factories: Pseudomonas putida KT2440, Halomonas bluephagenesis TD01, and Zymomonas mobilis ZM4. Curr Opin Biotechnol. 2025;92 - PubMed
-
- Chen G.Q., Jiang X.R. Next generation industrial biotechnology based on extremophilic bacteria. Curr Opin Biotechnol. 2018;50:94–100. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

