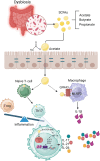
Commentary: Role of gut microbiota in infectious and inflammatory diseases
- PMID: 40756214
- PMCID: PMC12313634
- DOI: 10.3389/fmicb.2025.1613147
Commentary: Role of gut microbiota in infectious and inflammatory diseases
Keywords: gout; gut microbiota; hyperuricemia (HUA); inflammation; microbiome and dysbiosis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Comment on
-
Role of gut microbiota in infectious and inflammatory diseases.Front Microbiol. 2023 Mar 27;14:1098386. doi: 10.3389/fmicb.2023.1098386. eCollection 2023. Front Microbiol. 2023. PMID: 37051522 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources