Disparities in invasive pneumococcal disease, pneumonia, and otitis media among US children by comorbidity profile and insurance status
- PMID: 40756406
- PMCID: PMC12315112
- DOI: 10.3389/fpubh.2025.1558157
Disparities in invasive pneumococcal disease, pneumonia, and otitis media among US children by comorbidity profile and insurance status
Abstract
Background: Near-universal pediatric use of pneumococcal conjugate vaccines in the United States (US) has yielded substantive reductions childhood invasive pneumococcal disease (IPD), pneumonia (PNE), and otitis media (OM), especially among at-risk populations. We evaluated residual disparities in disease burden among US children by comorbidity profile and insurance type (as a proxy for socioeconomic status) during the post-PCV13 era.
Methods: We conducted a retrospective observational cohort study using two US healthcare claims databases: Optum Clinformatics DataMart (commercial) and Merative MarketScan Medicaid Multi-State Database. The two study populations comprised children aged <18 years and were stratified by age and comorbidity profile. Study outcomes included IPD, PNE, OM, and tympanostomy tube (TT) insertion, and were ascertained monthly during the follow-up period. Disease rates were expressed per 100,000 person-years, and age-specific relative rates were calculated by insurance type and comorbidity profile.
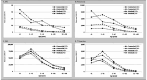
Results: Children with comorbidities aged <2 years had the highest rates of IPD and PNE, regardless of insurance status. Rates of IPD and PNE were also higher in children with Medicaid (vs. commercial) insurance; differences generally decreased with increasing age. Differences in incidence of OM and TT insertions between children with (vs. without) comorbidities were absent in the first 2 years of life but became apparent with increasing age.
Conclusion: Children with comorbidities and those with Medicaid insurance have a higher burden of IPD, PNE, and OM. Researchers should assess the impact that preventative strategies have on pediatric populations with the highest rates of disease to identify progress in achieving equity in health.
Keywords: Streptococcus pneumoniae; child; infections; otitis media; pneumonia.
Copyright © 2025 Lapidot, Averin, Weycker, Huang, Vietri, Arguedas, Cane, Lonshteyn, Rozenbaum and Pelton.
Conflict of interest statement
AC, LH, AdA, MR, and JV are employed by, and own stock in, Pfizer Inc. SP and RL received financial support from Pfizer Inc. for participation in study design, data analysis, and data interpretation. AhA, AL, and DW are employees of Avalere Health, which received funding from Pfizer in connection with this study and the development of this manuscript. This study was sponsored by Pfizer. The funder was involved with study concept, analysis; critical review of the manuscript; and the decision to submit the manuscript for publication. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Pneumococcal disease: surveillance and reporting Centers for Disease Control and Prevention (CDC) (2020). Available at: https://www.cdc.gov/pneumococcal/php/surveillance/index.html
-
- Hu T, Done N, Petigara T, Mohanty S, Song Y, Liu Q, et al. Incidence of acute otitis media in children in the United States before and after the introduction of 7- and 13-valent pneumococcal conjugate vaccines during 1998-2018. BMC Infect Dis. (2022) 22. doi: 10.1186/s12879-022-07275-9, PMID: - DOI - PMC - PubMed
-
- Lee YJ, Huang YT, Kim SJ, Kerpelev M, Gonzalez V, Kaltsas A, et al. Trends in invasive pneumococcal disease in cancer patients after the introduction of 7-valent pneumococcal conjugate vaccine: a 20-year longitudinal study at a major urban cancer center. Clin Infect Dis. (2018) 66:244–253. doi: 10.1093/cid/cix739, PMID: - DOI - PMC - PubMed
-
- Payne AB, Link-Gelles R, Fau-Azonobi I, Azonobi F-HWC, I, Hooper Wc Fau-Beall BW, Beall Bw Fau-Jorgensen JH, et al. Invasive pneumococcal disease among children with and without sickle cell disease in the United States, 1998 to 2009. Pediatr Infect Dis J. (2013) 32:1308. doi: 10.1097/INF.0b013e3182a11808 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical