A NAC family gene PmNAC32 associated with photoperiod promotes flower induction in Prunus mume
- PMID: 40756635
- PMCID: PMC12317187
- DOI: 10.1093/hr/uhaf157
A NAC family gene PmNAC32 associated with photoperiod promotes flower induction in Prunus mume
Abstract
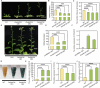
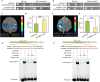
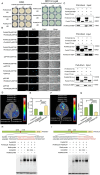
The photoperiod is essential to flower induction, and the exact timing of the process can be precisely regulated based on the relative duration of light and darkness. However, the mechanisms linking photoperiod and flower induction in woody plants remain largely unexplored. Using RNA-seq, we identified a photoperiod response factor PmNAC32, which is predominantly expressed in early-flowering varieties. Overexpression of PmNAC32 in Arabidopsis thaliana, tobacco, and Prunus mume calli resulted in accelerated flowering. Binding and activation analyses revealed that PmNAC32 can be directly suppressed by REVEILLE 1 (RVE1) and REVEILLE 3 (RVE3), implying that PmNAC32 plays a role in the photoperiodic signaling pathway. Further studies established that PmNAC32 functions as a positive regulator of CONSTANS-LIKE 5 (COL5) and a negative regulator of CONSTANS-LIKE 4 (COL4). Interestingly, we identified two homologs of PmNAC32, namely PmNAC29 and PmNAC47. These three proteins can interact with each other and enhance the regulation of PmCOL4 and PmCOL5. Although PmNAC29 and PmNAC47 can promote flower induction respectively, neither of them responded to the photoperiod. Thus, our results reveal a novel mechanism by which PmNAC32 regulates flower induction in Prunus mume.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nanjing Agricultural University.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Bäurle I, Dean C. The timing of developmental transitions in plants. Cell. 2006;125:655–64 - PubMed
-
- Putterill J, Laurie R, Macknight R. It’s time to flower: the genetic control of flowering time. BioEssays. 2004;26:363–73 - PubMed
-
- Tan F-C, Swain SM. Genetics of flower initiation and development in annual and perennial plants. Physiol Plant. 2006;128:8–17
-
- Kaur A, Maness N, Ferguson L. et al. Role of plant hormones in flowering and exogenous hormone application in fruit/nut trees: a review of pecans. Fruit Res. 2021;1:1–9
LinkOut - more resources
Full Text Sources

