Effect of Pharmacological Treatment on Exercise-Induced Bronchoconstriction and Allergic Inflammatory Response in Endurance Athletes
- PMID: 40756803
- PMCID: PMC12317798
- DOI: 10.26603/001c.141859
Effect of Pharmacological Treatment on Exercise-Induced Bronchoconstriction and Allergic Inflammatory Response in Endurance Athletes
Abstract
Background: Endurance athletes (EA) with lung disease and allergic inflammation have worse performance.
Purpose: To examine whether pharmacological treatment can reduce airway disorders such as exercise-induced bronchoconstriction (EIB) and allergic inflammatory response (AIR) in EA.
Study design: Prospective, controlled clinical trial.
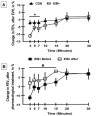
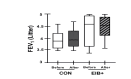
Methods: EA who were marathon runners underwent eucapnic voluntary hyperventilation (EVH) for screening assessment. EA who fulfilled the criteria for an EIB+ after an EVH were included in the treatment group (EIB+; n=13), and those who did not were included in the control group (CON; n=18). The athletes were assessed before and 30 days after the intervention. Outcomes included cardiopulmonary exercise testing, lung function, allergic symptoms (allergic questionnaire for athletes [AQUA©]), AIR (T helper [Th]-1, Th2, and Th17 lymphocytes in cell cultures), inflammatory mediator expression, salivary immunoglobulin (Ig)A, blood cortisol, blood IgE levels, and airway inflammation (fraction exhaled nitric oxide [FeNO]). Both groups were advised to keep the same training routine, and only the EIB+ received pharmacological treatment with inhaled corticosteroids (400-800 mcg/day) and long-acting bronchodilators (12 mcg/day). The CON and EIB+ groups underwent the same assessments after the intervention and were compared pre- and post-intervention, and effect sizes were calculated.
Results: EIB+ (males, age 28.1±7.4 years, BMI 20.3±1.0 kg/m2) CON (males, age 29.8±6.5 years, BMI 20.5±1.6 kg/m2) participated. At baseline, the O2 peak, lung function, allergic symptoms, IgE, IgA, FeNO levels, and AIR were not significancly different between groups (p>0.05). After pharmacological treatment, only the EIB+ group showed a decrease in EIB (p<0.001) and an increase in VO2peak compared to baseline (p<0.05). However, no difference was observed in the expression of inflammatory mediators (p>0.05).
Conclusion: Pharmacological treatment reduces EIB and increases the aerobic perforance/fitness in endurance athletes. These benefits occur without modification of the AIR.
Level of evidence: Level II- Prospective Comparative Study.
Keywords: allergy; lung; marathon; running; triathlon.
© The Author(s).
Figures
References
-
- Subjective responses to sprint interval exercise in adults with and without Exercise-induced bronchoconstriction. Good J., Dogra S. 2018J Asthma. 55(10):1059–1067. doi: 10.1080/02770903.2017.1391282. https://doi.org/10.1080/02770903.2017.1391282 - DOI - PubMed
-
- Effects of a heat and moisture exchanger on respiratory function and symptoms post-cold air exercise. Frischhut C., Kennedy M. D., Niedermeier M., Faulhaber M. Mar;2020 Scand J Med Sci Sports. 30(3):591–601. doi: 10.1111/sms.13603. https://doi.org/10.1111/sms.13603 - DOI - PMC - PubMed
-
- Exercise-induced bronchoconstriction: background, prevalence, and sport considerations. Bonini M., Silvers W. 2018Immunol Allergy Clin North Am. 38(2):205–214. doi: 10.1016/j.iac.2018.01.007. https://doi.org/10.1016/j.iac.2018.01.007 - DOI - PubMed
-
- Work Group Report: Perspectives in diagnosis and management of exercise-induced bronchoconstriction in athletes. Greiwe J., Cooke A., Nanda A.., et al. 2020J Allergy Clin Immunol Pract. 8(8):2542–2555. doi: 10.1016/j.jaip.2020.05.020. https://doi.org/10.1016/j.jaip.2020.05.020 - DOI - PubMed
-
- Differences in injury risk and characteristics of injuries between novice and experienced runners over a 4-year period. Kemler E., Blokland D., Backx F.., et al. 2018Phys Sportsmed. 46(4):485–491. doi: 10.1080/00913847.2018.1507410. https://doi.org/10.1080/00913847.2018.1507410 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
