Efficacy and safety of telitacicept in systemic lupus erythematosus with lupus nephritis and nephrotic syndrome: a 12-month retrospective cohort study
- PMID: 40756983
- PMCID: PMC12313615
- DOI: 10.3389/fphar.2025.1613790
Efficacy and safety of telitacicept in systemic lupus erythematosus with lupus nephritis and nephrotic syndrome: a 12-month retrospective cohort study
Abstract
Background: This retrospective cohort study evaluated the therapeutic efficacy and safety profile of telitacicept, a novel dual B-cell-activating factor (BAFF)/a proliferation-inducing ligand (April) inhibitor, in managing systemic lupus erythematosus (SLE) patients with lupus nephritis (LN) and nephrotic syndrome (NS), with particular focus on renal and hematological parameters.
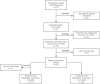
Methods: 12 SLE patients with biopsy-confirmed LN and NS who received weekly subcutaneous telitacicept (80/160 mg) combined with standard therapies for ≥12 months were analyzed. Primary endpoints include changes in Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores, 24-h urinary protein excretion (24hUpr), complement levels (Complement Component 3/Complement Component 4), anti-double-stranded DNA antibodies (anti-dsDNA) titers, immunoglobulin profiles, serum creatinine, and hemoglobin (HGB) at baseline, 3-month, and 12-month intervals. Statistical analysis was performed using SPSS 26.0 and R 4.1.2. The significance level was assessed using a one-sample t-test of the log ratios, with the null hypothesis assuming no effect.
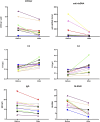
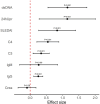
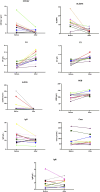
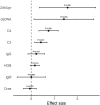
Results: Significant improvements were observed in the cohort (91.7% female, median age 30): SLEDAI: Median reduction from 13 to 4 (p = 0.0029), 24hUpr: 4.0 g/24 h → 0.83 g/24 h (p < 0.001), anti-dsDNA: 120 IU/mL → 13 IU/mL (p = 0.003), Complement restoration: C3 0.56→0.84 g/L; C4 0.1→0.22 g/L (both p < 0.001), HGB improvement: 110→120 g/L (p = 0.0144). Compared to 80 mg dose subgroup, the 160 mg dose subgroup (83.3%) showed superior outcomes with no severe adverse events.
Conclusion: Telitacicept demonstrates robust clinical efficacy in LN-NS management through dual B-cell regulation and complement restoration mechanisms. These practical findings support its potential as a targeted therapy for renal and hematological manifestations of SLE, requiring further validation through randomized controlled trials.
Keywords: efficacy; lupus nephritis; nephrotic syndrome; safety; telitacicept.
Copyright © 2025 Liu, Li, Li, Zhu, Lin, Xu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

