Luminescent Nanocomposite SiO2/EuTTA/ZIF‑8 Loaded with Uvaol: Synthesis, Characterization, Anti-Inflammatory Effects, and Molecular Docking Analysis
- PMID: 40757268
- PMCID: PMC12311653
- DOI: 10.1021/acsomega.5c04682
Luminescent Nanocomposite SiO2/EuTTA/ZIF‑8 Loaded with Uvaol: Synthesis, Characterization, Anti-Inflammatory Effects, and Molecular Docking Analysis
Abstract
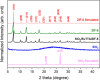
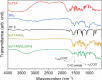
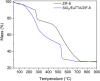
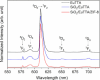
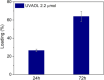
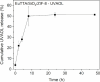
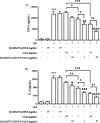
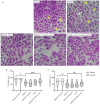
Asthma is a chronic inflammatory respiratory condition that requires innovative approaches for effective drug delivery. Uvaol, a natural triterpene with potent anti-inflammatory effects, holds promise for asthma treatment. However, its low bioavailability limits its therapeutic applications. To overcome this challenge, we synthesized a europium-based nanocomposite (SiO2/EuTTA/ZIF-8) to enhance uvaol delivery. The nanomaterials were characterized using UV-visible absorption spectroscopy, fluorescence analysis, and molecular docking simulations. Drug loading and release studies were conducted in PBS to evaluate encapsulation efficiency and controlled release properties. Cytotoxicity assays were performed to assess biocompatibility, and molecular docking was used to analyze interactions between uvaol and ZIF-8. The synthesized nanocomposite demonstrated efficient uvaol encapsulation and controlled release in PBS. Cytotoxicity assays revealed biocompatibility at low concentrations (≤10 μg/mL) and toxicity at higher concentrations (≥50 μg/mL). In addition, SiO2/EuTTA/ZIF-8-uvaol revealed the inhibition of the lipopolysaccharide (LPS)-induced secretion of IL-6 and TNF-α in J774 cells. Molecular docking studies highlighted hydrophobic interactions and π-π stacking between uvaol and ZIF-8, supporting stable drug-nanocarrier binding. These findings suggest that SiO2/EuTTA/ZIF-8-uvaol is a promising platform for improving uvaol bioavailability and enabling controlled drug delivery in asthma therapy. Additionally, europium luminescence offers the advantage of real-time monitoring, further enhancing the potential of this nanocomposite for therapeutic applications.
© 2025 The Authors. Published by American Chemical Society.
Figures






Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
A potent multifunctional ZIF-8 nanoplatform developed for colorectal cancer therapy by triple-delivery of chemo/radio/targeted therapy agents.J Mater Chem B. 2024 Jan 24;12(4):1096-1114. doi: 10.1039/d3tb02571c. J Mater Chem B. 2024. PMID: 38229578
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
