Design, Synthesis, and Biological Activity of Amine-Type Cyclopentanepyridinone Derivatives as HIV‑1 Non-Nucleoside Reverse Transcriptase Inhibitors
- PMID: 40757284
- PMCID: PMC12311863
- DOI: 10.1021/acsomega.5c03953
Design, Synthesis, and Biological Activity of Amine-Type Cyclopentanepyridinone Derivatives as HIV‑1 Non-Nucleoside Reverse Transcriptase Inhibitors
Abstract
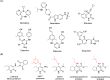
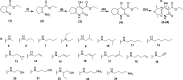
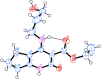
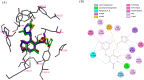
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) targeting human immunodeficiency virus (HIV) frequently exhibit suboptimal pharmacological properties and are often compromised by drug-resistant mutations. This underscores the ongoing need for the development of developing novel reverse transcriptase (RT) inhibitors. In this study, we report the synthesis of 20 novel amine-type cyclopentanepyridinone derivatives as NNRTIs. The chlorinated C-4 core was functionalized with various amines of differing chain lengths, yielding final products in 43-88% yields. Derivatives bearing alkyl and alkenyl chains at the C-4 position demonstrated anti-HIV activity at nanomolar to micromolar concentrations. Among them, compound 9 exhibited the most potent inhibitory activity against HIV and wild-type (WT) HIV-1 RT, with an EC50 of 540 nM and an IC50 of 33.89 μM, respectively, while maintaining low cytotoxicity (CC50 > 100 μM). Molecular docking analysis revealed interactions with key residues in the NNRTI binding pocket (NNIBP), including Lys101, Tyr181, Tyr188, and the conserved residues Phe227, Trp229, and Leu234, both in WT HIV-1 RT and Tyr188Leu HIV-1 RT. Moreover, molecular dynamics (MD) simulations with WT HIV-1 RT showed that compounds 6, 9, and 10 formed up to two hydrogen bonds with RT, supporting their ability to bind effectively within the NNIBP.
© 2025 The Authors. Published by American Chemical Society.
Figures


Similar articles
-
Identification of a novel small-molecule inhibitor of the HIV-1 reverse transcriptase activity with a non-nucleoside mode of action.Virol J. 2025 Mar 7;22(1):65. doi: 10.1186/s12985-025-02680-3. Virol J. 2025. PMID: 40055750 Free PMC article.
-
Efavirenz or nevirapine in three-drug combination therapy with two nucleoside or nucleotide-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naïve individuals.Cochrane Database Syst Rev. 2016 Dec 10;12(12):CD004246. doi: 10.1002/14651858.CD004246.pub4. Cochrane Database Syst Rev. 2016. PMID: 27943261 Free PMC article.
-
Co-formulated abacavir-lamivudine-zidovudine for initial treatment of HIV infection and AIDS.Cochrane Database Syst Rev. 2013 Mar 28;2013(3):CD005481. doi: 10.1002/14651858.CD005481.pub3. Cochrane Database Syst Rev. 2013. PMID: 23543540 Free PMC article.
-
Structure-based design of diarylpyrimidines and triarylpyrimidines as potent HIV-1 NNRTIs with improved metabolic stability and drug resistance profiles.J Med Virol. 2024 Mar;96(3):e29502. doi: 10.1002/jmv.29502. J Med Virol. 2024. PMID: 38450817
-
Further exploring the tolerant region II: Identification of 2,4,5-trisubstituted pyrimidines as HIV-1 reverse transcriptase allosteric inhibitors with desirable antiviral activities and reduced cytotoxicity.Eur J Med Chem. 2025 Nov 5;297:117992. doi: 10.1016/j.ejmech.2025.117992. Epub 2025 Jul 21. Eur J Med Chem. 2025. PMID: 40706448
References
-
- SeyedAlinaghi S., Afsahi A. M., Moradi A., Parmoon Z., Habibi P., Mirzapour P., Dashti M., Ghasemzadeh A., Karimi E., Sanaati F., Hamedi Z., Molla A., Mehraeen E., Dadras O.. Current ART, Determinants for Virologic Failure and Implications for HIV Drug Resistance: An Umbrella Review. AIDS Res. Ther. 2023;20(1):74. doi: 10.1186/s12981-023-00572-6. - DOI - PMC - PubMed
-
- Eggleton, J. S. ; Nagalli, S. . Highly Active Antiretroviral Therapy (HAART); StatPearls: Treasure Island, FL, 2023. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
