Enzymatic Halogenation of Silk Fibroin from
- PMID: 40757296
- PMCID: PMC12311704
- DOI: 10.1021/acsomega.5c03545
Enzymatic Halogenation of Silk Fibroin from
Abstract
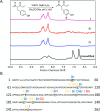
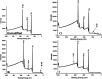
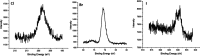
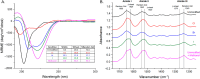
Silk fibroin from the silkworm, , is a unique biomaterial that has been extensively studied for a variety of applications that utilize robust mechanical properties, biological compatibility, and controlled self-assembly properties. This study tested carbon-halogen (C-X) bond halogenation to alter the chemical composition of silk fibroin with the intention to generate novel functional materials. In brief, silk fibroin side-chain modification used halogen salts (NaX, X = Cl, Br, and I), hydrogen peroxide (H2O2), and the vanadium-dependent haloperoxidase from to produce primarily halogenated tyrosine residues along the amorphous regions of the silk fibroin protein. Halogenation was confirmed with various methods, including 1D 1H NMR, X-ray photoelectron spectroscopy, and analysis of chymotrypsin peptide digests by Q-TOF Liquid Chromatography-Mass Spectrometry. Secondary structure analysis by FTIR-ATR, circular dichroism, and Raman spectroscopy revealed increase in helical conformation of solubilized halogenated silk fibroin, while dried film functionality demonstrated higher abundance of β-sheet structures by maintenance of random coil content. Evaluation by contact angle measurement demonstrated increased hydrophilicity on silk fibroin films following addition of halogens by supporting the formation of water insoluble hydrogels after treatment with various organic and salt solvents. This study is the first to characterize the effects of enzymatic halogenations on the properties of silk fibroin, and this post-translational modification will be useful for the addition of non-natural small molecules or ligands, introducing new material types afforded by the silk fibroin structure.
© 2025 The Authors. Published by American Chemical Society.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Silk-Ovarioids: establishment and characterization of a human ovarian primary cell 3D-model system.Hum Reprod Open. 2025 Jul 10;2025(3):hoaf042. doi: 10.1093/hropen/hoaf042. eCollection 2025. Hum Reprod Open. 2025. PMID: 40799620 Free PMC article.
-
An innovative treatment for hypoxic-ischemic encephalopathy: Silk fibroin nanomaterials improve neural stem cell axon formation and facilitate cognitive improvement.Neural Regen Res. 2025 Jun 19. doi: 10.4103/NRR.NRR-D-24-01178. Online ahead of print. Neural Regen Res. 2025. PMID: 40537000
-
Silk fibroin-based dressings with antibacterial and anti-inflammatory properties.Eur J Pharm Sci. 2024 Apr 1;195:106710. doi: 10.1016/j.ejps.2024.106710. Epub 2024 Jan 26. Eur J Pharm Sci. 2024. PMID: 38281552
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
