The Role of c‑Jun Signaling in Cytidine Analog-Induced Cell Death in Melanoma
- PMID: 40757321
- PMCID: PMC12311696
- DOI: 10.1021/acsomega.5c02807
The Role of c‑Jun Signaling in Cytidine Analog-Induced Cell Death in Melanoma
Abstract
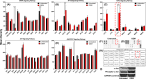
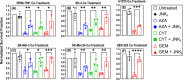
Melanoma stands as an increasingly pressing health concern. Enhanced mitochondrial metabolism has been reported in melanoma cells that survived treatment with traditional therapeutics, including cytidine analogs like gemcitabine (GEM). These findings suggest that chemotherapeutic drugs may play dual roles in promoting both cell survival and cell death, although the underlying mechanisms require further investigation. Herein, we conducted proteomics analysis on GEM-treated melanoma cells and found a drug-induced activation of DNA damage response and apoptosis, along with cell cycle arrest. Additionally, GEM treatment significantly altered protein networks related to mitochondrial ribosomal activity, the electron transport chain, and translation. Furthermore, we reported an upregulation of the JNK/c-Jun network in connection with the apoptotic proteins. Co-treatment with a Jun N-terminal Kinase (JNK) inhibitor, JNK-IN-8 (JNKi), significantly increased cell survival, suggesting the involvement of c-Jun signaling in GEM-induced cell death. Additionally, proteomics analysis revealed that JNKi downregulated apoptosis in cotreated cells, highlighting the potential role of the JNK/c-Jun network inhibition in chemotherapeutic tolerance. Collectively, our findings bridge gaps in understanding how melanoma cells respond to cytidine analogs by demonstrating the multifaceted effects of these agents in (1) inducing JNK-mediated apoptotic cell death and (2) promoting a state of cell cycle inhibition.
© 2025 The Authors. Published by American Chemical Society.
Figures



Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
