5-HT7 antagonists confer analgesia via suppression of neurotrophin overproduction in submucosal nerves of mouse models with visceral hypersensitivity
- PMID: 40757652
- PMCID: PMC12400787
- DOI: 10.1113/JP286444
5-HT7 antagonists confer analgesia via suppression of neurotrophin overproduction in submucosal nerves of mouse models with visceral hypersensitivity
Abstract
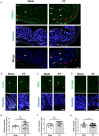
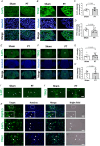
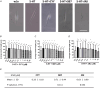
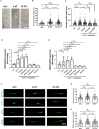
Dysregulated serotonin/5-hydroxytryptamine (5-HT) metabolism and dense mucosal neurite distribution are associated with visceral hypersensitivity (VH), which plays a key role in irritable bowel syndrome (IBS) pain symptoms. The 5-HT receptor subtype 7 (5-HT7) is involved in neuroplasticity. We aim to investigate the analgesic effects of 5-HT7 antagonists in mouse models and explore downstream changes of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) in the enteric neurons. Selective 5-HT7 antagonists [CYY1005 (CYY), JNJ-18038683 (JNJ) or SB269970 (SB7)] were orogavaged to an IBS-like mouse model of postinflammatory VH after resolution from trinitrobenzene sulfonic acid-induced colitis. Visceromotor response, mucosal neurite outgrowth, and neurotrophin levels were assessed. Orogavage of CYY had stronger analgesic effects than JNJ or SB7 in the IBS-like mice. Higher density of 5-HT7-expressing mucosal nerve fibres was associated with increased NGF and BDNF immunostaining in the submucosal plexus of IBS-like mice compared to those of sham mice. No difference in the serotonergic neurons of spinal ganglia and brain regions was observed between IBS-like and sham mice. Moreover, CYY treatment for 10 days decreased the colonic neurotrophin levels and reduced pain sensation in IBS-like mice. Serotonin-induced neurite elongation was inhibited by 5-HT7 antagonists in mouse primary submucosal neuron cultures and human SH-SY5Y cell lines. Neutralizing antibodies to neurotrophins also diminished the serotonin-induced neurite outgrowth. Lastly, 5-HT7 activation upregulated neurotrophin expression in neurons via Cdk5/mTOR/Cdc42 signalling. In conclusion, 5-HT7 antagonists attenuated neurotrophin overproduction in the submucosal nerve plexus, leading to lesser mucosal innervation and reduced intestinal nociception in IBS-like mouse models. KEY POINTS: Aberrant serotonin/5-hydroxytryptamine (5-HT) metabolism and dense mucosal neurites are linked with irritable bowel syndrome (IBS) pain symptoms. Current treatments are ineffective for IBS pain management. Previous studies showed 5-HT receptor subtype 7 (5-HT7)-expressing nerve fibres in the colonoscopic biopsy of IBS patients and colonic mucosa of IBS-like mouse models. Moreover, 5-HT7 activation is involved in neuroplasticity in neural cell lines in vitro. Although visceral pain has been studied extensively in spinal afferents, the involvement of enteric neurons in intestinal nociception remains to be determined. Orogavage of 5-HT7 antagonist reduced mucosal neurite outgrowth and decreased neurotrophin overproduction in submucosal nerves, resulting in lower intestinal pain. Activation of 5-HT7 promotes neurite elongation in primary submucosal nerve cultures. The findings implicate a potential role of submucosal plexus in 5-HT7-dependent visceral hypersensitivity.
Keywords: colon hyperalgesia; enteric nervous system; irritable bowel syndrome; neuroendocrine; nociceptive afferent; primary neuron cultures; serotonergic nerve; submucosal plexus.
© 2025 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Balemans, D. , Aguilera‐Lizarraga, J. , Florens, M. V. , Jain, P. , Denadai‐Souza, A. , Viola, M. F. , Alpizar, Y. A. , Van Der Merwe, S. , Vanden Berghe, P. , Talavera, K. , Vanner, S. , Wouters, M. M. , & Boeckxstaens, G. E. (2019). Histamine‐mediated potentiation of transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 4 signaling in submucosal neurons in patients with irritable bowel syndrome. American Journal of Physiology‐Gastrointestinal and Liver Physiology, 316(3), G338‐G349. - PubMed
-
- Barbara, G. , Wang, B. , Stanghellini, V. , de Giorgio, R. , Cremon, C. , Di Nardo, G. , Trevisani, M. , Campi, B. , Geppetti, P. , Tonini, M. , Bunnett, N. W. , Grundy, D. , & Corinaldesi, R. (2007). Mast cell‐dependent excitation of visceral‐nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology, 132(1), 26–37. - PubMed
MeSH terms
Substances
Grants and funding
- NSTC 113-2320-B002-062-MY3/National Science and Technology Council
- NSTC 111-2622-B-002-016/National Science and Technology Council
- NSTC 113-2823-8-002-002/National Science and Technology Council
- MOST 110-2622-B-002-013/National Science and Technology Council
- MOST 110-2320-B-002-011-MY3/National Science and Technology Council
LinkOut - more resources
Full Text Sources
Miscellaneous
