Inhibition Effects of Patchouli Alcohol, Carvacrol, p-Cymene, Eucalyptol and Their Formulations Against Influenza Virus Pneumonia Through TLR4/NF-κB/NLRP3 Signaling Pathway
- PMID: 40757669
- PMCID: PMC12320473
- DOI: 10.1111/cbdd.70150
Inhibition Effects of Patchouli Alcohol, Carvacrol, p-Cymene, Eucalyptol and Their Formulations Against Influenza Virus Pneumonia Through TLR4/NF-κB/NLRP3 Signaling Pathway
Abstract
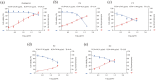
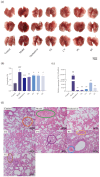
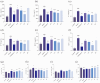
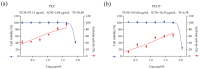
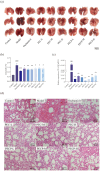
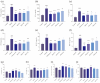
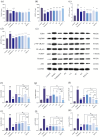
As a kind of drug mostly used historically to treat epidemics, aromatic botanicals have volatile oils as active components. The study aims to evaluate the anti-influenza viral pneumonia effects of volatile monomers patchouli alcohol (PA), carvacrol (CV), p-Cymene (PC), eucalyptol (EC) and their formulations from various aspects through the influenza virus A/PR/8/34 (H1N1) infection experiment in vivo and in vitro and carry out in-depth studies on the anti-inflammatory mechanisms. In this study, we found that all four volatile monomers mentioned above could exert antiviral effects by suppressing pulmonary viral load and lung index and improving lung lesions in mice with influenza pneumonia. In addition, elevated levels of cytokines and chemokines in the serum were suppressed, the proportion of T-lymphocytes in the peripheral blood was altered, and antioxidative stress indices were improved, whose mechanism of action related to anti-inflammation, possibly acting on the Toll-Like Receptor 4/Nuclear Factor-κB/nucleotide-binding domain leucine-rich repeat and pyrin domain-containing receptor 3 (TLR4/NF-κB/NLRP3) pathway. The study provides an experimental basis for volatile monomers and their formulations of aromatic herbs for treating influenza virus pneumonia.
Keywords: TLR4/NF‐κB/NLRP3 signaling pathway; antiviral activity; carvacrol; eucalyptol; inflammation; influenza virus type a; patchouli alcohol; p‐cymene.
© 2025 The Author(s). Chemical Biology & Drug Design published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
The mechanism of action in Mussaenda pubescens (Yuye Jinhua) against influenza A virus: Evidence from in vitro and in vivo studies.Phytomedicine. 2025 Sep;145:157070. doi: 10.1016/j.phymed.2025.157070. Epub 2025 Jul 13. Phytomedicine. 2025. PMID: 40684492
-
Liu Shen Wan inhibits influenza a virus and excessive virus-induced inflammatory response via suppression of TLR4/NF-κB signaling pathway in vitro and in vivo.J Ethnopharmacol. 2020 Apr 24;252:112584. doi: 10.1016/j.jep.2020.112584. Epub 2020 Jan 21. J Ethnopharmacol. 2020. PMID: 31972325
-
Integrated In Silico, In Vitro, and In Vivo Studies Reveal Mangiferin as a Promising Antiviral Agent Against H1N1/pdm2009 Influenza Virus.Viruses. 2025 Jun 21;17(7):873. doi: 10.3390/v17070873. Viruses. 2025. PMID: 40733492 Free PMC article.
-
Neuraminidase inhibitors for preventing and treating influenza in adults and children.Cochrane Database Syst Rev. 2014 Apr 10;2014(4):CD008965. doi: 10.1002/14651858.CD008965.pub4. Cochrane Database Syst Rev. 2014. PMID: 24718923 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
References
-
- Alfonso‐Loeches, S. , Ureña‐Peralta J. R., Morillo‐Bargues M. J., Oliver‐De La Cruz J., and Guerri C.. 2014. “Role of Mitochondria ROS Generation in Ethanol‐Induced NLRP3 Inflammasome Activation and Cell Death in Astroglial Cells.” Frontiers in Cellular Neuroscience 8: 216. 10.3389/fncel.2014.00216. - DOI - PMC - PubMed
-
- Ao, H. , Liu H., Wang J., et al. 2016. “Determination of Volatile Oil by GC‐MS and Evaluation of Heavy Metals Residue in Fructus Amomi From Different Producing Areas.” Traditional Chinese Drug Research and Clinical Pharmacology 27, no. 2: 250–254.
-
- Aoken, A. , Wu T., Bai X., and Maitinuer M.. 2021. “Comparative Study of Chemical Composition and Biological Activity of Essential Oil From Four Species of Mentha L. Plants Growing in Xinjiang by GC‐MS.” Food Research and Development 42, no. 8: 127–131.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

