Short-Term Outcomes of Pediatric Patients With Mild Autosomal Recessive RPE65-Associated Retinal Dystrophy Treated With Voretigene Neparvovec
- PMID: 40757766
- PMCID: PMC12327541
- DOI: 10.1167/tvst.14.8.8
Short-Term Outcomes of Pediatric Patients With Mild Autosomal Recessive RPE65-Associated Retinal Dystrophy Treated With Voretigene Neparvovec
Abstract
Purpose: Voretigene neparvovec is approved for RPE65-associated inherited retinal degeneration (RPE65-IRD) in the United States and the European Union. According to current knowledge, early treatment benefits efficacy. However, consensus on treating mild cases is lacking due to ambiguity in balancing clinical benefits with potential side effects. Therefore, we present short-term outcomes of four pediatric patients with milder types of RPE65-IRD.
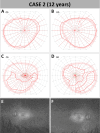
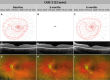
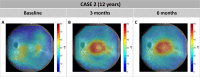
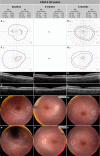
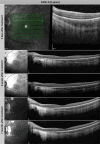
Methods: Two unrelated pediatric patients were unilaterally treated at the University Eye Hospital in Tübingen, Germany. Another two unrelated pediatric patients were bilaterally treated at Ghent University Hospital, Belgium. Examinations were performed before and until at least 6 months after treatment, including best-corrected visual acuity, slit-lamp examination, fundus photography, short-wavelength fundus autofluorescence, optical coherence tomography, 90° kinetic perimetry, dark-adapted chromatic perimetry, and full-field stimulus threshold measurements.
Results: Despite surgical challenges, treatment with voretigene neparvovec was successful in all four patients. All patients showed rod functional rescue with stable best-corrected visual acuity. Three patients suffered chorioretinal atrophy at the retinotomy site but none developed signs of fast-growing CRA. One case developed limited CRA in the bleb area, potentially related to inflammation in the subretinal space.
Conclusions: Treatment with voretigene neparvovec was safe and effective in patients with mild RPE65-IRD. Early treatment showed good functional outcomes. Also, treatment at stages without profound retinal degeneration might lower the risk of fast-growing CRA.
Translational relevance: This study aids clinical decision-making in unclear cases by demonstrating that early treatment with voretigene neparvovec in mild RPE65-IRD provides functional benefits while minimizing the risk of fast-growing chorioretinal atrophy.
Conflict of interest statement
Disclosure:
Figures


References
-
- Gu SM, Thompson DA, Srikumari CR, et al.. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997; 17: 194–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

