Low-Dose Hydrocortisone in Cirrhotic Patients With Septic Shock: A Double-Blind Randomised Placebo-Controlled Trial
- PMID: 40757786
- PMCID: PMC12320571
- DOI: 10.1111/liv.70257
Low-Dose Hydrocortisone in Cirrhotic Patients With Septic Shock: A Double-Blind Randomised Placebo-Controlled Trial
Abstract
Background and aims: Steroid supplementation remains controversial in septic shock, partly due to the heterogeneity of the populations studied. Cirrhotic patients with sepsis frequently develop relative adrenal insufficiency, showing poor response to vasopressors and poor prognosis. Early administration of low-dose hydrocortisone might facilitate shock reversal and reduce mortality in this population.
Methods: Double-blind, randomised, placebo-controlled multicentre trial, enrolling adult cirrhotic patients with septic shock. Patients were assigned to receive i.v. hydrocortisone (100 mg followed by a continuous infusion of 200 mg/24 h) or placebo, for at least 3 days, followed by a tapering period of 3-7 days, depending on the time of shock resolution. Primary endpoint was 28-day all-cause mortality.
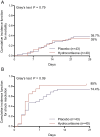
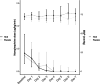
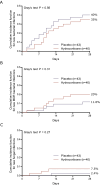
Results: After enrolment of 83 patients the trial was stopped early due to slow inclusions. Most patients required low-to-moderate doses of vasopressors. There was no difference in 28-day mortality (35% vs. 39.5%; p = 0.84) between hydrocortisone and placebo groups. Shock resolution (85% vs. 72.1%; p = 0.25) and days to shock resolution [3 days (2.2-4; IQR) vs. 4 (2-7.5; IQR)] were also similar between groups. More patients receiving placebo died of refractory shock (47.6% vs. 8.7%). No significant differences between treatment arms were observed in shock relapse, new shock episodes or bacterial or fungal superinfections during hospital stay. Hypo- and hyperglycaemias were more frequent in the hydrocortisone arm. Severity of ACLF at inclusion and inadequacy of empirical antibiotic therapy (HR = 6.40; 95% CI: 3.21-12.79) independently predicted 28-day mortality.
Conclusions: Supplemental hydrocortisone did not improve short-term survival in cirrhotic patients with septic shock requiring low-to-moderate doses of vasopressors.
Trial registration: S-number University Hospitals Leuven, Belgium: S55168; EUDRACT: 2010-024273-38; Clinicaltrials.gov id: NCT02602210.
Keywords: ACLF; mortality; refractory shock; safety; shock reversal.
© 2025 The Author(s). Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Annane D., Pastores S. M., Rochwerg B., et al., “Guidelines for the Diagnosis and Management of Critical Illness‐Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017,” Intensive Care Medicine 43 (2017): 1751–1763. - PubMed
-
- Sprung C. L., Annane D., Keh D., et al., “Hydrocortisone Therapy for Patients With Septic Shock,” New England Journal of Medicine 358 (2008): 111–124. - PubMed
-
- Annane D., Sébille V., Charpentier C., et al., “Effect of Treatment With Low Doses of Hydrocortisone and Fludrocortisone on Mortality in Patients With Septic Shock,” Journal of the American Medical Association 288 (2002): 862–871. - PubMed
-
- Venkatesh B., Finfer S., Cohen J., et al., “Adjunctive Glucocorticoid Therapy in Patients With Septic Shock,” New England Journal of Medicine 378 (2018): 797–808. - PubMed
-
- Annane D., Renault A., Brun‐Buisson C., et al., “Hydrocortisone Plus Fludrocortisone for Adults With Septic Shock,” New England Journal of Medicine 378 (2018): 809–818. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

