Development of AI Based Fibrosis Detection Algorithm by SHG/TPEF Microscopy for Fully Quantified Liver Fibrosis Assessment in MASH
- PMID: 40757802
- PMCID: PMC12320568
- DOI: 10.1111/liv.70258
Development of AI Based Fibrosis Detection Algorithm by SHG/TPEF Microscopy for Fully Quantified Liver Fibrosis Assessment in MASH
Abstract
Background and aims: Metabolic dysfunction-associated steatotic liver disease (MASLD) is a major global cause of chronic liver disease, with the potential to progress from steatosis to metabolic dysfunction-associated steatohepatitis (MASH) and cirrhosis. Fibrosis is a key determinant of liver-related morbidity and mortality, highlighting the need for precise, reproducible assessment methods. This study aimed to develop and validate an Artificial Intelligence (AI)-based fibrosis detection algorithm using Second Harmonic Generation/Two Photon Excitation Fluorescence (SHG/TPEF) microscopy.
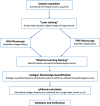
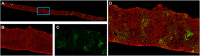
Methods: The algorithm integrates SHG/TPEF microscopy, which uses ultra-fast lasers to capture intrinsic optical signals from unstained liver biopsies, with Machine Learning (ML)-based image analysis. The resulting qFibrosis model quantifies collagen morphology to generate a continuous fibrosis index.
Results: A standardised workflow was established, encompassing sample acquisition, SHG/TPEF imaging, region-specific analysis and collagen feature quantification. Each step of the AI-based ML of qFibrosis algorithm used to assess and quantify liver fibrosis is described in detail in this study.
Conclusions: This AI-driven approach enables accurate, continuous quantification of liver fibrosis, overcoming the variability of traditional histopathology. The qFibrosis model has potential as a standardised tool for therapeutic evaluation and disease monitoring in MASLD/MASH, representing a significant advancement in liver fibrosis assessment.
Keywords: MASH; MASLD; Machine Learning; artificial intelligence; fibrosis.
© 2025 The Author(s). Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
M.N. reports consulting agreements and research support from several pharmaceutical companies but none represent a potential conflict for this paper. K.A., R.Y. and D.T. are employees of HistoIndex Pte Ltd., Singapore. P.B. is an employee of Rectify Pharmaceuticals Inc.
Figures



References
-
- Younossi Z. M., Koenig A. B., Abdelatif D., Fazel Y., Henry L., and Wymer M., “Global Epidemiology of Nonalcoholic Fatty Liver Disease: Meta‐Analytic Assessment of Prevalence, Incidence, and Outcomes,” Hepatology 64 (2016): 73–84. - PubMed
-
- Younossi Z., Anstee Q. M., Marietti M., et al., “Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention,” Nature Reviews. Gastroenterology & Hepatology 15 (2018): 11–20. - PubMed
-
- Charlton M. R., Burns J. M., Pedersen R. A., Watt K. D., Heimbach J. K., and Dierkhising R. A., “Frequency and Outcomes of Liver Transplantation for Nonalcoholic Steatohepatitis in the United States,” Gastroenterology 141 (2011): 1249–1253. - PubMed
-
- Wong R. J., Cheung R., and Ahmed A., “Nonalcoholic Steatohepatitis Is the Most Rapidly Growing Indication for Liver Transplantation in Patients With Hepatocellular Carcinoma in the U.S,” Hepatology 59 (2014): 2188–2195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

