Modeling of hepatitis B virus infection spread in primary human hepatocytes
- PMID: 40757857
- PMCID: PMC12455953
- DOI: 10.1128/jvi.00927-25
Modeling of hepatitis B virus infection spread in primary human hepatocytes
Abstract
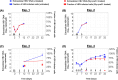
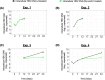
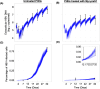
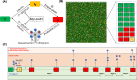
Chronic hepatitis B virus (HBV) infection poses a significant global health threat, causing severe liver diseases including cirrhosis and hepatocellular carcinoma. We characterized HBV DNA kinetics in primary human hepatocytes (PHHs) over 32 days post-inoculation (p.i.) and modified our in-vivo agent-based modeling (ABM) to gain insights into the HBV lifecycle and spread in vitro. Parallel PHH cultures were mock-treated or treated with HBV entry inhibitor Myr-preS1 (6.25 µg/mL) was initiated 24 h p.i. In untreated PHH, three viral DNA kinetic patterns were identified: (i) an initial decline, followed by (ii) rapid amplification and (iii) slower amplification/accumulation. In the presence of Myr-preS1, viral DNA and infected cell numbers in phase 3 were effectively blocked, with minimal to no increase. This suggests that phase 2 represents viral amplification in initially infected cells, while phase 3 corresponds to viral spread to naïve cells. The ABM reproduced well the HBV kinetic patterns observed and predicted that the viral eclipse phase lasts between 18 and 38 h. After the eclipse phase, the viral production rate increased over time, starting with a slow production cycle of 1 virion per day, which gradually accelerated to 1 virion per hour after 3 days. Approximately 4 days later, virion production reached a steady state production rate of 4 virions/h. The estimated median efficacy of Myr-preS1 in blocking HBV spread was 91% (range: 90-92%). The HBV kinetics and the predicted estimates of the HBV eclipse phase duration and HBV production cycles in PHH are similar to those predicted in uPA/SCID mice with human livers.IMPORTANCEWhile primary human hepatocytes (PHHs) are the most physiologically relevant culture system for studying HBV infection in vitro, a comprehensive understanding of HBV infection kinetics and spread in PHH is lacking. In this study, we characterize HBV viral kinetics and modify our in vivo agent-based modeling (ABM) to provide quantitative insights into the HBV production cycle and viral spread in PHH. The ABM provides an estimate of the HBV eclipse phase duration, HBV production cycles, and Myr-preS1 efficacy in blocking HBV spread in PHH. The results resemble those predicted in uPA/SCID mice with human livers, demonstrating that estimated HBV infection kinetic parameters in PHH in vitro mirror those observed in the in vivo HBV infection chimeric mouse model.
Keywords: Myr-preS1 treatment; agent-based modeling; hepatitis B virus; primary human hepatocytes; viral hepatitis.
Conflict of interest statement
Yuji Ishida and Chise Tateno are employees of PhoenixBio Co.
Figures


Update of
-
Modeling of hepatitis B virus infection spread in primary human hepatocytes.bioRxiv [Preprint]. 2025 Feb 7:2025.02.05.636596. doi: 10.1101/2025.02.05.636596. bioRxiv. 2025. Update in: J Virol. 2025 Sep 23;99(9):e0092725. doi: 10.1128/jvi.00927-25. PMID: 39975229 Free PMC article. Updated. Preprint.
References
-
- World Health Organization . 2024. Hepatitis B. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

