Premature termination of DNA Damage Repair by 3-Methyladenine potentiates cisplatin cytotoxicity in nasopharyngeal carcinoma cells
- PMID: 40758730
- PMCID: PMC12321125
- DOI: 10.1371/journal.pone.0329272
Premature termination of DNA Damage Repair by 3-Methyladenine potentiates cisplatin cytotoxicity in nasopharyngeal carcinoma cells
Abstract
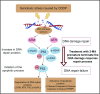
3-Methyladenine (3-MA) is widely recognized as a PI3K inhibitor involved in autophagy regulation. However, it is also a byproduct of DNA damage repair, and its role in modulating DNA damage response (DDR) mechanisms remains largely unexplored. Cisplatin (CDDP), a cornerstone chemotherapeutic agent for nasopharyngeal carcinoma (NPC), exerts its cytotoxic effects by inducing DNA damage in tumor cells. This study investigates the combined effects of CDDP and 3-MA on NPC cells. Cell viability and the half-maximal inhibitory concentration (IC50) were assessed using the Cell Counting Kit-8 (CCK-8) assay. Flow cytometry was employed to analyze cell cycle distribution, mitochondrial membrane potential (MMP) alterations, and apoptosis. γ-H2AX foci formation and morphological changes were examined via fluorescence microscopy, while Western blotting was used to evaluate proteins associated with the DNA damage response. The combination treatment significantly reduced cell viability and lowered the IC50 compared to CDDP alone. While both treatments induced Sub-G1 phase arrest, the combination resulted in greater MMP loss and apoptosis. Western blot analysis further revealed that 3-MA enhanced CDDP cytotoxicity by suppressing ATM/ATR/p53-mediated DNA damage repair and promoting apoptotic signaling. These findings suggest that 3-MA sensitizes NPC cells to CDDP by disrupting DNA repair processes, offering a promising therapeutic strategy.
Copyright: © 2025 Zhou et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

