HERV-derived epitopes represent new targets for T-cell-based immunotherapies in ovarian cancer
- PMID: 40759443
- PMCID: PMC12320039
- DOI: 10.1136/jitc-2024-010099
HERV-derived epitopes represent new targets for T-cell-based immunotherapies in ovarian cancer
Abstract
Background: Ovarian cancer represents the most lethal gynecological cancer with poor response to checkpoint inhibitors. Human endogenous retroviruses (HERVs) are aberrantly expressed by tumor cells and may represent a source of shared T-cell epitopes for cancer immunotherapy regardless of the tumor mutational burden.
Methods: A transcriptomic analysis based on RNA sequencing was developed to quantify the expression of HERV-K sequences containing the selected epitopes. The presence of HERV-K/HML-2 Gag antigen was then assessed by immunohistochemistry (IHC) on tumor microarrays from ovarian cancer samples and normal ovarian tissues. A specific immunopeptidomics approach was developed to detect epitopes on human leukocyte antigens (HLA) molecules. Epitope-specific CD8+ T cells were quantified by multimer staining. HERV-specific T cells were obtained after in vitro stimulation of T cells from HLA-A2-positive healthy donors or patients with ovarian cancer, and in vitro target cell killing was evaluated using real-time analysis. In vivo antitumor efficacy of HERV-specific T cells was assessed in an avian embryo model.
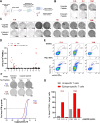
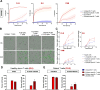
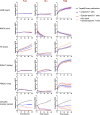
Results: Epitope-containing HERV transcripts were significantly higher in ovarian cancers compared with normal tissues. The presence of the HERV-K/HML-2 Gag antigen was confirmed by IHC in 20/40 (50%) ovarian cancers while no Gag expression was found in normal ovarian tissue samples. Immunopeptidomics analysis revealed the presence of epitopes on HLA molecules on the surface of ovarian tumor cell lines but not on normal primary cells from critical tissues. Low percentages of HERV-specific T cells were detected among tumor-infiltrating lymphocytes from ovarian cancers. Furthermore, in vitro stimulation of patient T cells induced functional epitope-specific T cells, confirming the immunogenicity of these epitopes in patients with ovarian cancer. In vitro, HERV-specific T cells specifically killed ovarian cancer cells in an HLA class I-restricted manner while sparing normal HLA-A2-positive primary cells derived from critical tissues. Epitope-specific CD8+ T cells exhibited a strong antitumoral activity in vivo, inducing a highly significant decrease in tumor volume in comparison with control groups.
Conclusion: These results provide the preclinical rationale for developing T-cell-based approaches against HERV-K-derived epitopes in ovarian cancer.
Keywords: Immunotherapy; Ovarian Cancer; T cell.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: PB, JV-G, SD are co-inventors on a patent application filed on the subject matter of this study (WO-2020049169-A1). PB, OT, YE, VM, MD, SM, CD, EE, NC, SD are employees of ErVimmune. SD is founder and chairman of ErVimmune.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
