An affinity-modulated T cell engager targeting Claudin 18.2 shows potent anti-tumor activity with limited cytokine release
- PMID: 40759445
- PMCID: PMC12323529
- DOI: 10.1136/jitc-2025-011857
An affinity-modulated T cell engager targeting Claudin 18.2 shows potent anti-tumor activity with limited cytokine release
Abstract
Background: AZD5863 is a bispecific T cell engager (TCE) with high affinity to CLDN18.2 and low affinity to cluster of differentiation 3 (CD3), designed to decrease its peripheral cytokine release potential, improve the therapeutic index, and maintain potent anti-tumor activity.
Methods: AZD5863 was evaluated using CLDN18.2-expressing human cell lines alone or in co-cultures with human or cynomolgus monkey peripheral blood mononuclear cells to determine affinities, specificity, potency, and bystander killing activity. In vivo, AZD5863-mediated tumor growth inhibition and pharmacodynamics were evaluated in humanized mice or human CD3 transgenic mice implanted with CLDN18.2-expressing cancer cell lines.
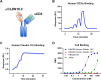
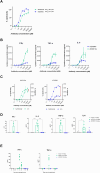
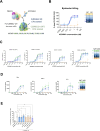
Results: AZD5863 was shown to bind specifically to human and cynomolgus monkey CLDN18.2 and to CD3, with CLDN18.2 binding also conserved against the murine protein. AZD5863 mediated T cell-dependent anti-tumor activity against CLDN18.2-expressing lines, with potency significantly correlating with CLDN18.2 receptor density. Cytokine secretion induced by AZD5863, in vitro and in vivo, was lower compared with a CLDN18.2 TCE with higher affinity for CD3. AZD5863 mediated T cell-dependent bystander killing of CLDN18.2-negative cells in the presence of CLDN18.2-expressing cells, in a mechanism partly dependent on interferon gamma (IFNγ), tumor necrosis factor alpha (TNFα), and Fas ligand. In vivo, AZD5863 treatment resulted in potent tumor control in pancreatic, gastric, and esophageal models and enhanced engraftment of immune populations in a humanized model.
Conclusions: AZD5863 mediates potent anti-tumor activity in vitro and in vivo, while inducing limited levels of cytokines. This work improves our understanding of the mechanism of action of affinity balanced TCEs and informs the design of a phase 1 trial testing AZD5863 in gastric, pancreatic, and esophageal adenocarcinoma (NCT06005493).
Keywords: Bispecific T cell engager - BiTE; Cytokine release syndrome; Gastric Cancer.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: Miguel Gaspar, Marina Natoli, Laure Castan, Sharif Rahmy, Martin Korade III, Cathryn Kelton, Kathy Mulgrew, Oisin Huhn, D. Gareth Rees, Anna Sigurdardottir, Christopher Lloyd, Jonathan Jonas Taylor, Phillip M. Brailey, Laura Dallaway, Aleksandra Toloczko, Nicolas Giraldo, Maria A.S. Broggi, Andrew Kunihiro, Sudhanshu Abhishek, Jim Eyles, Kathryn Ball, Jonathan Fitzgerald, Scott A. Hammond, Saso Cemerski, Simon J. Dovedi, and Mark Cobbold are employees of and have ownership interest (including patents) in AstraZeneca. Yun He and Yiping Rong are employees of and have ownership interest (including patents) in Harbour BioMed.
Figures


References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
