Cancer therapy and cachexia
- PMID: 40759570
- PMCID: PMC12321403
- DOI: 10.1172/JCI191934
Cancer therapy and cachexia
Abstract
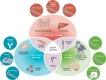
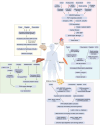
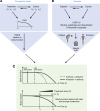
A central challenge in cancer therapy is the effective delivery of anticancer treatments while minimizing adverse effects on patient health. The potential dual impact of therapy is clearly illustrated in cancer-associated cachexia, a multifactorial syndrome characterized by involuntary weight loss, systemic inflammation, metabolic dysregulation, and behavioral alterations such as anorexia and apathy. While cachexia research often focuses on tumor-driven mechanisms, the literature indicates that cancer therapies themselves, particularly chemotherapies and targeted treatments, can initiate or exacerbate the biological pathways driving this syndrome. Here, we explore how therapeutic interventions intersect with the pathophysiology of cachexia, focusing on key organ systems including muscle, adipose tissue, liver, heart, and brain. We highlight examples such as therapy-induced upregulation of IL-6 and growth-differentiation factor 15, both contributing to reduced nutrient intake and a negative energy balance via brain-specific mechanisms. At the level of nutrient release and organ atrophy, chemotherapies also converge with cancer progression, for example, activating NF-κB in muscle and PKA/CREB signaling in adipose tissue. By examining how treatment timing and modality align with the natural trajectory of cancer cachexia, we underscore the importance of incorporating physiological endpoints alongside tumor-centric metrics in clinical trials. Such integrative approaches may better capture therapeutic efficacy while preserving patient well-being.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

