Lactate signalling leads to aggregation of immune-inflammatory hotspots and SLC5A12 blockade promotes their resolution
- PMID: 40759752
- PMCID: PMC12373510
- DOI: 10.1038/s42255-025-01331-9
Lactate signalling leads to aggregation of immune-inflammatory hotspots and SLC5A12 blockade promotes their resolution
Abstract
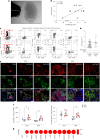
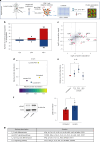
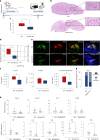
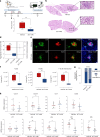
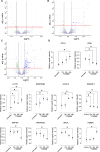
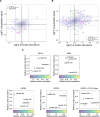
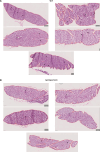
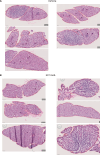
Ectopic lymphoid structures (ELS) are aggregates of lymphoid cells that often form within inflamed tissues in patients with autoimmune diseases, cancer, infectious diseases and cardiovascular conditions. These structures drive B cell maturation into memory B cells and plasma cells through B cell and T cell co-stimulation, and their role in pathogenesis is increasingly recognized. Understanding how ELS develop and persist in inflamed tissues is essential for elucidating the pathogenesis and treatment responses in diseases in which they have a prominent role. Here we show that metabolic pathways and specific metabolites, in particular lactate, are master regulators of ELS organization in Sjögren's disease (SjD), the second-most common autoimmune rheumatic disease. Furthermore, inhibiting lactate uptake by lactate transporters, specifically by SLC5A12 blockade, represents a previously unappreciated checkpoint in autoimmune inflammatory diseases. This approach results in multidimensional pro-resolution effects, including reduced inflammatory cytokine levels, enhanced T cell egress from inflamed sites and diminished T cell and B cell areas and their segregation within ELS.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.M. is a founder and CSO of Solute Guard Therapeutics (SGTx). M.B. is a member of the scientific advisory board of SGTx. The other authors declare no competing interests.
Figures





References
-
- Bombardieri, M., Lewis, M. & Pitzalis, C. Ectopic lymphoid neogenesis in rheumatic autoimmune diseases. Nat. Rev. Rheumatol.13, 141–154 (2017). - PubMed
-
- Pitzalis, C., Jones, G. W., Bombardieri, M. & Jones, S. A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol.14, 447–462 (2014). - PubMed
-
- Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature577, 556–560 (2020). - PubMed
-
- Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature577, 561–565 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

