Histone lactylation regulates DOCK4 to control heat nociception and supports Dynein-mediated Nav1.7 trafficking
- PMID: 40759894
- PMCID: PMC12322138
- DOI: 10.1038/s41467-025-62343-3
Histone lactylation regulates DOCK4 to control heat nociception and supports Dynein-mediated Nav1.7 trafficking
Abstract
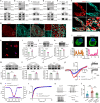
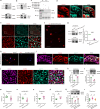
Heat nociception involves thermosensors like transient receptor potential channel V1 in dorsal root ganglion (DRG) neurons, but their loss only partially impairs heat sensing, suggesting other mechanisms. Autism frequently involves abnormal pain perception, but its mechanisms remain unclear. Here we show that dedicator of cytokinesis 4 (Dock4), an autism susceptibility gene, is decreased in DRG neurons across multiple pain models via histone H4K8 lactylation. DOCK4 deficiency in sensory neurons increases heat nociception in mice. Mechanistically, DOCK4 interacts with sodium channel Nav1.7 and mediates its trafficking from the membrane to the cytoplasm in DRG neurons. Acting an adaptor protein, DOCK4 binds the motor protein Dynein to form a Dynein/DOCK4/Nav1.7 complex, where Dynein provides the mechanical force for Nav1.7 trafficking. DOCK4 knockdown in sensory neurons also enhances heat nociception in male nonhuman primates. Thus, the Dynein/DOCK4/Nav1.7 complex represents a thermosensor-independent mechanism regulating heat nociception and provides insights into abnormal pain in autism.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Sharma, S. R., Gonda, X. & Tarazi, F. I. Autism spectrum disorder: Classification, diagnosis and therapy. Pharm. Ther.190, 91–104 (2018). - PubMed
-
- Lord, C., Cook, E. H., Leventhal, B. L. & Amaral, D. G. Autism spectrum disorders. Neuron28, 355–363 (2000). - PubMed
-
- Robertson, C. E. & Baron-Cohen, S. Sensory perception in autism. Nat. Rev. Neurosci.18, 671–684 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

