Characterizing sarcopenia and sarcopenic obesity in patients aged 65 years and over, at risk of mobility disability: a multicenter observational trial (SARA-OBS)
- PMID: 40759914
- PMCID: PMC12323141
- DOI: 10.1186/s12877-025-05895-9
Characterizing sarcopenia and sarcopenic obesity in patients aged 65 years and over, at risk of mobility disability: a multicenter observational trial (SARA-OBS)
Abstract
Background: Aging is associated with a progressive change of body composition characterized by muscle mass decline and accumulation of adipose tissue that can lead to sarcopenia and obesity, respectively. The prevalence of sarcopenia is poorly known given the different parameters and thresholds in proposed definitions. The combination of obesity (defined as a percentage of body fat mass of > 25% in men and > 35% in women) and sarcopenia (SO) adds complexity to the characterization of this pathology. SARA-OBS aimed to better characterize sarcopenia (including SO) and its consequences on physical function over time, in community-dwelling older adults at risk of mobility disability, and to support the design of further interventional clinical trials.
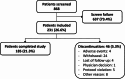
Methods: This was an international, multicenter, 6-month observational study of men and women aged ≥ 65 years suffering from sarcopenia according to the Foundation for the National Institute of Health (FNIH) cut-offs for Sarcopenia and with a Short Physical Performance Battery (SPPB) ≤ 8. The primary endpoint was the change in Gait Speed (GS) in the 400-meter walking test (400MWT), reported at baseline and at Month 6/ end of the study (EOS). Secondary endpoints included changes in handgrip strength (HGS), physical performance (6-Minute Walking Distance [6MWD], SPPB), the Physical Function Domain (PF-10) sub-score and total score of the SF-36 survey and the Sarcopenia and Quality of Life (SarQoL) questionnaire.
Results: Overall, the mean (± SD) change from baseline to Month 6/EOS in 400MWT GS was - 0.027 ± 0.171 m/sec (p = 0.064). Both GS and 6MWD decreased significantly in subgroup with GS ≥ 0.8 m/sec at baseline (-0.047 ± 0.185 m/sec; p = 0.017 and - 24.01 ± 68.24 m; p = 0.001, respectively). In subgroup with SPPB = 8 at baseline, 6MWD also decreased (-36.80 ± 67.60 m; p < 0.001). We observed a significant change from baseline for 6MWD in the SO subgroup (-18.30 ± 81.95 m; p = 0.013). Neither HGS nor SarQoL changed significantly from baseline to Month 6/EOS.
Conclusions: SARA-OBS results contribute to defining subgroups of older adults at risk of functional decline over 6 months, specifically subjects with SPPB = 8, affecting GS and the 6MWD. Additionally, the SO subpopulation exhibited a relevant deterioration in physical function as evaluated by the 6MWD.
Trial registration: NCT03021798 (ClinicalTrials.gov). Date of registration 16/01/2017.
Keywords: Mobility disability; Older adults; Physical performance; Sarcopenia; Sarcopenic obesity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was submitted and approved by local and central Ethics Committees in France (Comité de Protection de Personnes Sud-Ouest & Outre-Mer IV), Belgium (Comité d’Ethique Hospitalo-Facultaire Universitaire de Liège), Italy (Comitato Etico dell’Universita SAPIENZA and dell’Universita Campus Bio-Medico Di Roma) and the US (Western Institutional Review Board-Copernicus group and Columbia University Institutional Review Board 2). We certify that this study has received approvals from the appropriate ethical committees as described above. Written informed consent were obtained from the patient or legal authorized representative. Consent for publication: Not applicable. Competing interests: RAF reports grant support from Lonza, Biophytis, National Institutes of Health, and USDA, scientific advisory board membership for Biophytis, Amazentis, Inside Tracker, Rejuventate Biomed, Aging in Motion, consultancies for Embion, Biophytis, Amazentis, Pfizer, Nestle, Rejuvenate Biomed.YR reports support from CHU Toulouse, University Paul Sabatier and INSERM CERPOP1295 (employee), to be a shareholder of SARQOL SPRL, a spin-off of the University of Liege, consultancy fees from Longeveron, to be a Scientific Advisory Board member to Biophytis, and have received honoraria for lectures for Pfizer.OB reports to be stakeholder of SARQOL SRL, a spin off of the University of Liege in charge of the interest of the SarQoL and reports consulting or lecture fees (in the last 5 years) from Amgen, Aptissen, Biophytis, IBSA, Mylan, Novartis, Nutricia, Orifarm, Sanofi, UCB and Viatris.CT, RVM, SV and WD are employees of Biophytis SA.JM is the President of the Scientific Advisory Board of Biophytis SA.CM and SDS are former employees of Biophytis.AT, LMD, MB, MD, MR, RAI declare that they have no competing interests. YR reports support from CHU Toulouse, University Paul Sabatier and INSERM CERPOP1295 (employee), to be a shareholder of SARQOL SPRL, a spin-off of the University of Liege, consultancy fees from Longeveron, to be a Scientific Advisory Board member to Biophytis, and have received honoraria for lectures for Pfizer. OB reports to be stakeholder of SARQOL SRL, a spin off of the University of Liege in charge of the interest of the SarQoL and reports consulting or lecture fees (in the last 5 years) from Amgen, Aptissen, Biophytis, IBSA, Mylan, Novartis, Nutricia, Orifarm, Sanofi, UCB and Viatris. CT, RVM, SV and WD are employees of Biophytis SA. JM is the President of the Scientific Advisory Board of Biophytis SA. CM and SDS are former employees of Biophytis. AT, LMD, MB, MD, MR, RAI declare that they have no competing interests.
References
-
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–5. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical