Artificial liver support with Cytosorb and continuous veno-venous hemodiafiltration versus advanced organ support (ADVOS) for critically ill patients with hyperbilirubinemia and acute-on-chronic liver failure (ACLF)
- PMID: 40759919
- PMCID: PMC12323257
- DOI: 10.1186/s12882-025-04342-6
Artificial liver support with Cytosorb and continuous veno-venous hemodiafiltration versus advanced organ support (ADVOS) for critically ill patients with hyperbilirubinemia and acute-on-chronic liver failure (ACLF)
Abstract
Background: As many as 30% of critically ill patients in intensive care units experience acute liver dysfunction with hyperbilirubinemia as a part of multiorgan failure that is associated with poor outcome. This retrospective cohort study was aimed at comparing CytoSorb and ADVOS in terms of bilirubin removal and overall survival among critically ill patients with hyperbilirubinemia ≥ 7 mg/dL.
Methods: At the University Hospital Essen, between January 2021 and March 2024, 71 patients were treated with CytoSorb integrated in a continuous veno-venous hemodiafiltration (CVVHDF) circuit, and 71 patients were treated with ADVOS. Each therapy session lasted 24 h. We separately analyzed the subgroup of patients with acute-on-chronic liver failure (ACLF), in which 31 patients were treated with CytoSorb and 66 patients were treated with ADVOS.
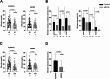
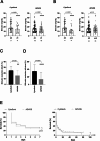
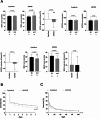
Results: The first single sessions of both CytoSorb with CVVHDF and ADVOS were associated with a statistically significant decrease in total serum bilirubin levels (Cytosorb, 20 to 14 mg/dL, p < 0.0001; ADVOS, 16 to 14 mg/dL, p < 0.0001), but the percentage bilirubin reduction was more pronounced for CytoSorb treatment (26% vs. 17%, p = 0.0002). The number of days of treatment was similar for both groups (3 vs. 4, p = 0.07). After completion of therapy, serum levels of total bilirubin had decreased significantly; 19.9 to 11.3 mg/dl (p < 0.0001) in the CytoSorb group and 16.3 to 14.0 mg/dL (p = 0.003) in the ADVOS group. The relative bilirubin reduction was significantly higher after application of CytoSorb than after treatment with ADVOS (35% (IQR 19,54) vs. 15% (IQR - 11;54), p < 0.0001). Regarding patients with ACLF, relative reduction of bilirubin after the first session as well as after the completion of liver support was significantly higher among patients who were treated with CVVHDF and CytoSorb than among those patients who received ADVOS. The relative removal of creatinine and urea nitrogen was significantly higher after ADVOS treatment than after CytoSorb with CVVHDF treatment considering all critically ill patients as well as ACLF patients. Seven-day or in-hospital mortality rates were high among critically ill patients and patients with ACLF in both liver support groups.
Conclusions: Our results showed that CytoSorb and CVVHDF treatment performed better than ADVOS in bilirubin removal among critically ill patients with hyperbilirubinemia caused by acute liver dysfunction and in the subgroup of patients with ACLF. ADVOS was more efficient in eliminating creatinine and urea nitrogen than was CVVHDF with CytoSorb. Additional prospective randomized controlled trials are warranted to investigate the efficacy of hemoperfusion with CytoSorb for liver disease indications among critically ill patients.
Clinical trial number: Not applicable.
Keywords: ADVOS; Acute-on-chronic liver failure; Bilirubin; Continuous veno-venous hemodiafiltration; CytoSorb; Secondary acquired liver dysfunction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the University Hospital Essen (23-11563-BO, 23-11170-BO). The consent to participate was waived by the local ethics committee of the University Duisburg-Essen (23-11563-BO, 23-11170-BO) due to the retrospective analyses that deal with data obtained from clinical routine. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Jonsdottir S, Arnardottir MB, Andresson JA, Bjornsson HK, Lund SH, Bjornsson ES. Prevalence, clinical characteristics and outcomes of hypoxic hepatitis in critically ill patients. Scand J Gastroenterol. 2022;57(3):311–8. - PubMed
-
- Grek A, Arasi L. Acute liver failure. AACN Adv Crit Care. 2016;27(4):420–9. - PubMed
-
- Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Bllod Purif. 2002;20(3):252–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

