Predicting head and neck cancer response to radiotherapy with a chemokine-based model
- PMID: 40760079
- PMCID: PMC12322276
- DOI: 10.1038/s41598-025-13346-z
Predicting head and neck cancer response to radiotherapy with a chemokine-based model
Abstract
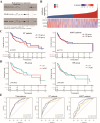
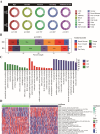
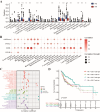
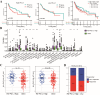
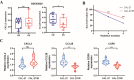
Radiotherapy resistance remains a major challenge in Head and neck squamous cell carcinoma (HNSCC) treatment. This study aimed to develop a chemokine-based model for predicting radiosensitivity in HNSCC using a retrospective analysis of 432 patients from the TCGA database. We identified a model incorporating CXCL2, CCL28, and CCR8 expression that effectively stratified patients into radiosensitive (RS) and radioresistant (RR) groups. Patients in the RS group demonstrated significantly improved overall survival (OS) with radiotherapy, whereas this prognostic advantage was not observed in the non-radiotherapy group. Notably, patients within the RS group with high PD-L1 expression exhibited even better OS and increased immune infiltration, indicating a synergistic relationship between radiosensitivity and PD-L1 expression. Further analyses revealed enrichment of immune-related pathways and higher effector immune cell abundance in the RS group, suggesting greater potential for immunotherapy response. Corroborating these findings, analysis of the GSE40020 cohort showed significant upregulation of CCL28 in patients with complete response compared to those with post-treatment failure. In vitro experiments using radiosensitive and radioresistant Tongue squamous cell carcinoma (TSCC) cell lines validated the association between chemokine gene expression and radiosensitivity. Our model provides a valuable tool for identifying HNSCC patients who may benefit from combined treatment strategies incorporating synergistic anti-tumor agents.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Siegel, R. L., Giaquinto, A. N., Jemal, A. & Cancer statistics CA: a cancer journal for clinicians 74, 12–49 (2024). (2024). 10.3322/caac.21820 - PubMed
-
- Bhatia, A. & Burtness, B. Treating head and neck cancer in the age of immunotherapy: A 2023 update. Drugs83, 217–248. 10.1007/s40265-023-01835-2 (2023). - PubMed
-
- Tang, E. et al. Treatment strategy for distant synchronous metastatic head and neck squamous cell carcinoma. Curr. Oncol. Rep.21, 102. 10.1007/s11912-019-0856-5 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

