The Parkinson's drug benztropine possesses histamine receptor 1-dependent host-directed antimicrobial activity against Mycobacterium tuberculosis
- PMID: 40760081
- PMCID: PMC12322000
- DOI: 10.1038/s44259-025-00143-x
The Parkinson's drug benztropine possesses histamine receptor 1-dependent host-directed antimicrobial activity against Mycobacterium tuberculosis
Abstract
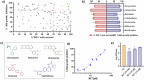
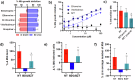
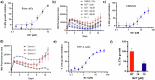
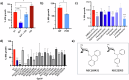
Intracellular pathogens such as Mycobacterium tuberculosis (Mtb) evade host defence mechanisms to infect and survive within host cells. Host-directed therapy (HDT) offers a promising alternative to antibiotics and may overcome antimicrobial resistance. Using high-content screening, we identified benztropine (BZT), an approved Parkinson's disease drug, as a potent inhibitor of intracellular Mtb. BZT is active in both human and murine macrophages but is inactive in broth. In an aerosol Mtb mouse infection model, oral administration of BZT reduced the burden of Mtb in the lungs by up to 70%. BZT was also active against Salmonella enterica serovar Typhimurium (STm) in an abscess model of infection, significantly reducing size and bacterial load. Chemical competition assays, CRISPR knockouts, and siRNA silencing assays revealed that BZT's activity against Mtb is mediated via macrophage histamine receptor 1 (HRH1). Our findings establish BZT as a promising repurposed candidate and a lead compound for developing HRH1-targeting antibacterial HDTs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics: The mouse Mtb and STm infection trials were conducted in accordance with Canadian Council on Animal Care (CCAC) guidelines and approved by the University of Saskatchewan Animal Research Ethics Board (Protocol #20230084) and the University of British Columbia Animal Care Committee (Protocol A23-0030-A002), respectively.
Figures


References
-
- WHO. Global Tuberculosis Reporthttps://www.who.int/teams/global-programme-on-tuberculosis-and-lung-heal... (2024).
Grants and funding
LinkOut - more resources
Full Text Sources

