Insulin DEgludec/LIraglutide versus multiple daily insulin injections in the transition from hospital to outpatient management assessed by continuous glucose monitoring: the DELI transition trial
- PMID: 40760249
- PMCID: PMC12361261
- DOI: 10.1007/s00125-025-06446-y
Insulin DEgludec/LIraglutide versus multiple daily insulin injections in the transition from hospital to outpatient management assessed by continuous glucose monitoring: the DELI transition trial
Abstract
Aims/objective: The aim of the study was to assess the safety profile (defined as the percentage of patients with at least one hypoglycaemic event [more than 15 min with glucose levels <3.0 mmol/l as documented by continuous glucose monitoring] in the first 4 weeks of follow-up) for insulin degludec/liraglutide (IDegLira) compared with multiple daily insulin injections (MDI) during the transition from hospital to an outpatient setting.
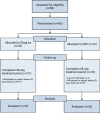
Methods: The study was an open-label, randomised, controlled clinical trial comparing IDegLira to MDI after hospital discharge in patients with type 2 diabetes. The study evaluated the percentage of patients with at least one hypoglycaemic event, the hypoglycaemia event density, the time in range (TIR 3.8-10 mmol/l), the time below range (TBR <3.0 or <3.8 mmol/l), and other glycaemic management metrics measured by continuous glucose monitoring.
Results: Sixty-four patients were included in the analysis (32 in each group). They had a baseline HbA1c of 103 ± 11.6 mmol/mol (11.6 ± 1.7%) and age of 58 ± 12.4 years (means ± SD). The proportion of patients with at least one hypoglycaemic event (plasma glucose <3.0 mmol/l) was lower in the IDegLira group than in the MDI group (6.2% vs 31.3%; p<0.010), as was the hypoglycaemia event density (incidence rate ratio 15.2; 95% CI 6.2, 48.2; p<0.001), TBR <3.8 mmol/l (0.9% vs 2.9%; p=0.019) and TBR <3.0 mmol/l (0.6% vs 1.3%, p=0.008). The TIR 3.8-10 mmol/l was higher in the IDegLira group (80.6% vs 69.7%; p=0.008). The findings were consistent regardless of baseline HbA1c.
Conclusions/interpretation: IDegLira proved to be safer and more effective than MDI for individuals with type 2 diabetes who had suboptimal glycaemic control, aiding in their transition from hospital to outpatient care.
Trial registration: Clinicaltrials.gov NCT05767255 FUNDING: This research was funded by a grant from the Asociación Colombiana de Endocrinología, Diabetes y Metabolismo (ACE).
Keywords: Colombia; Hospital to home transition; IDegLira; Type 2 diabetes mellitus.
© 2025. The Author(s).
Conflict of interest statement
Acknowledgements: Medical writing in English and editorial assistance were provided by Festina Lente (Medellin, Colombia) and funded by Novo Nordisk Colombia. The collaboration agreement between the Pontificia Universidad Javeriana and Springer Nature enabled the open access publication. The authors assume full responsibility for the content and conclusions expressed in this manuscript. Novo Nordisk did not influence the content of this publication nor participate in the study design, data collection, analysis, interpretation or review, or in any stage of the publication process. Data availability: The datasets generated or analysed for the current study are available upon reasonable request from the sponsor institution (Hospital Universitario San Ignacio). Funding: Open Access funding provided by Colombia Consortium. This research was funded by a grant from the Asociación Colombiana de Endocrinología, Diabetes y Metabolismo (ACE). Authors’ relationships and activities: AMG-M received speaker fees from Novo Nordisk, Astra Zeneca, Sanofi, Eli Lilly, Boehringer Ingelheim, Abbott and Medtronic. DCH-C received speaker fees from Novo Nordisk, Sanofi and Abbott. The remaining authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work. Contribution statement: AMG-M conceived the study. AMG-M, DCH-C, LPV-C, OMM-V, CAY, YG-Q and PA designed the study, analysed the data and critically reviewed the protocol. SNC and CEP-N participated in the acquisition of data and database elaboration. All authors contributed to the final version of the manuscript, and approved the version to be published. AMG-M is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
Similar articles
-
Hybrid closed-loop systems for managing blood glucose levels in type 1 diabetes: a systematic review and economic modelling.Health Technol Assess. 2024 Dec;28(80):1-190. doi: 10.3310/JYPL3536. Health Technol Assess. 2024. PMID: 39673446 Free PMC article.
-
Once-weekly IcoSema versus multiple daily insulin injections in type 2 diabetes management (COMBINE 3): an open-label, multicentre, treat-to-target, non-inferiority, randomised, phase 3a trial.Lancet Diabetes Endocrinol. 2025 Jul;13(7):556-567. doi: 10.1016/S2213-8587(25)00052-X. Epub 2025 Jun 4. Lancet Diabetes Endocrinol. 2025. PMID: 40482670 Clinical Trial.
-
Glucose-lowering agents for treating pre-existing and new-onset diabetes in kidney transplant recipients.Cochrane Database Syst Rev. 2017 Feb 27;2(2):CD009966. doi: 10.1002/14651858.CD009966.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jul 30;8:CD009966. doi: 10.1002/14651858.CD009966.pub3. PMID: 28238223 Free PMC article. Updated.
-
Once-weekly insulin efsitora alfa versus once-daily insulin glargine U100 in adults with type 2 diabetes treated with basal and prandial insulin (QWINT-4): a phase 3, randomised, non-inferiority trial.Lancet. 2025 Jun 28;405(10497):2290-2301. doi: 10.1016/S0140-6736(25)01069-4. Epub 2025 Jun 22. Lancet. 2025. PMID: 40562048 Clinical Trial.
-
Dipeptidyl-peptidase (DPP)-4 inhibitors and glucagon-like peptide (GLP)-1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk for the development of type 2 diabetes mellitus.Cochrane Database Syst Rev. 2017 May 10;5(5):CD012204. doi: 10.1002/14651858.CD012204.pub2. Cochrane Database Syst Rev. 2017. PMID: 28489279 Free PMC article.
References
-
- Pasquel FJ, Urrutia MA, Cardona S et al (2021) Liraglutide hospital discharge trial: a randomized controlled trial comparing the safety and efficacy of liraglutide versus insulin glargine for the management of patients with type 2 diabetes after hospital discharge. Diabetes Obes Metab 23(6):1351–1360. 10.1111/dom.14347 - PMC - PubMed
-
- Donihi AC (2017) Practical recommendations for transitioning patients with type 2 diabetes from hospital to home. Curr Diab Rep 17(7):52. 10.1007/s11892-017-0876-1 - PubMed
-
- Galindo RJ, Moazzami B, Scioscia MF et al (2023) A randomized controlled trial comparing the efficacy and safety of IDegLira versus basal-bolus in patients with poorly controlled type 2 diabetes and very high HbA1c ≥9–15%: DUAL HIGH trial. Diabetes Care 46(9):1640–1645. 10.2337/dc22-2426 - PMC - PubMed
-
- Lingvay I, Pérez Manghi F, García-Hernández P et al (2016) Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA 315(9):898–907. 10.1001/jama.2016.1252 - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous