Ultraprocessed or minimally processed diets following healthy dietary guidelines on weight and cardiometabolic health: a randomized, crossover trial
- PMID: 40760353
- PMCID: PMC12532614
- DOI: 10.1038/s41591-025-03842-0
Ultraprocessed or minimally processed diets following healthy dietary guidelines on weight and cardiometabolic health: a randomized, crossover trial
Abstract
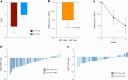
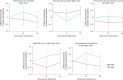
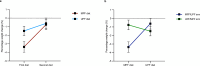
Ultraprocessed food (UPF) consumption is associated with noncommunicable disease risk, yet no trial has assessed its health impact within the context of national dietary guidelines. In a 2 × 2 crossover randomized controlled feeding trial, 55 adults in England (body mass index ≥25 to <40 kg m-2, habitual UPF intake ≥50% kcal day-1) were provided with two 8-week ad libitum diets following the UK Eatwell Guide: (1) minimally processed food (MPF) and (2) UPF, in a random order. Twenty-eight people were randomized to MPF then UPF, and 27 to UPF then MPF; 50 participants comprised the intention-to-treat sample. The primary outcome was the within-participant difference in percent weight change (%WC) between diets, from baseline to week 8. Participants were blinded to the primary outcome. MPF (%WC, -2.06 (95% confidence interval (CI), -2.99, -1.13) and UPF (%WC, -1.05 (95% CI, -1.98, -0.13)) resulted in weight loss, with significantly greater %WC on MPF (Δ%WC, -1.01 (95% CI, -1.87, -0.14), P = 0.024; Cohen's d, -0.48 (95% CI, -0.91, -0.06)). Mild gastrointestinal adverse events were common on both diets. Findings indicate greater weight loss on MPF than UPF diets and needing dietary guidance on food processing in addition to existing recommendations. Clinicaltrials.gov registration: NCT05627570 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.J.D. receives royalties from Amazon for a self-published book that mentions UPF, payments from Red Pen Reviews as a contributor, consultancy work for Consensus and Androlabs and travel fees from a USDA National Institute of Food and Agriculture grant, (AFRI project 1033399) for a workshop on food processing classifications. R.L.B. reports honoraria from Novo Nordisk, Eli Lilly, Medscape, ViiV Healthcare and International Medical Press and advisory board and consultancy work for Novo Nordisk, Eli Lilly, Pfizer, Gila Therapeutics, Epitomee Medical and ViiV Healthcare and from May 2023 is an employee and shareholder of Eli Lilly and Company. A.B. declares researcher-led grants from Novo Nordisk and honoraria from Novo Nordisk, Lilly, Office of Health Improvement and Disparity, Johnson and Johnson and Obesity UK outside the submitted work and is on the Medical Advisory Board and shareholder of Reset Health Clinics Ltd. C.A.M.G.W.-K. is a shareholder in Queen Square Analytics. J.M. reports institutional funding from Novo Nordisk, Rhythm Pharmaceuticals and Innovate UK outside the submitted work. C.v.T. receives royalties for a book on UPF and has been paid for other broadcasting on this subject (podcast and BBC documentaries). B.N. reports honoraria from Cook Medical, and research support from Aqua Medical. The other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
- MR/N013867/1/RCUK | Medical Research Council (MRC)
- MR/S026088/1/RCUK | Medical Research Council (MRC)
- PGL21/10079/Rosetrees Trust
- Research and Innovation Action Grants Human Brain Project 945539 (SGA3))/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- SP/F/20/150002/British Heart Foundation (BHF)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

