Dried Blood Spots and Plasma Separation Cards can Broaden Access to Molecular Testing for HBV, HCV and HIV
- PMID: 40760468
- PMCID: PMC12322363
- DOI: 10.1002/rmv.70059
Dried Blood Spots and Plasma Separation Cards can Broaden Access to Molecular Testing for HBV, HCV and HIV
Abstract
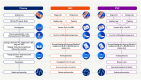
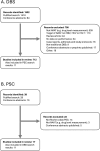
Approximately 3.5 million people acquired HBV, HCV, or HIV-1 in 2022, and 340 million persons are infected with at least one of these three viruses, mostly in low- and middle-income countries (LMICs). Nucleic acid amplification tests (NAAT) are precise and sensitive tools for viral infection diagnosis, monitoring and sequencing. NAATs typically rely on plasma specimens, which can be challenging to collect, store and transport in LMICs, limiting the reach of NAAT testing in rural and other resource constrained settings where trained phlebotomists and/or a cold chain for preservation and shipping are not available. Alternative specimen types that can overcome this limitation include dried blood spots (DBS) and dried plasma prepared using the Cobas Plasma Separation Card (PSC). We performed a literature review of DBS and PSC use for NAAT of HBV, HCV, or HIV-1 to summarise their performance characteristics, relative advantages and disadvantages and potential to support expanded access to NAAT. DBS have been used extensively for HIV-1 diagnosis and viral load monitoring, as well as for HBV and HCV, albeit to a much lesser degree. Compared to plasma, DBS perform well in terms of accuracy but have lower sensitivity. There is a risk of low specificity due to the presence of cellular nucleic acids in DBS and a resulting over-estimation of viral load. The PSC has similar accuracy and sensitivity compared to DBS, but improved specificity due to the removal of cellular components. Both DBS and PSC have the potential to enhance access in populations where the use of plasma is challenging.
© 2025 The Author(s). Reviews in Medical Virology published by John Wiley & Sons Ltd.
Figures


References
-
- World Health Organization . “Implementing the Global Health Sector Strategies on HIV, Viral Hepatitis and Sexually Transmitted Infections,” 2022–2030. Accessed, December 10, 2024, https://www.who.int/publications/i/item/9789240094925.
-
- World Health Organization . “Global Hepatitis Report 2024: Action for Access in Low‐ and Middle‐Income Countries”. Accessed, 2024, https://www.who.int/publications/i/item/9789240091672.
-
- Frank T. D., Carter A., Jahagirdar D., et al., “Global, Regional, and National Incidence, Prevalence, and Mortality of HIV, 1980–2017, and Forecasts to 2030, for 195 Countries and Territories: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017,” Lancet HIV 6, no. 12 (2019): e831–e859, 10.1016/s2352-3018(19)30196-1. - DOI - PMC - PubMed
-
- European Centre for Disease Prevention and Control . “Prevention of Hepatitis B and C in the EU/EEA”. Accessed, December 10, 2024, https://www.ecdc.europa.eu/assets/Prevention‐Hepatitis‐B‐and‐C/index.html.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

