Performance and comparison of FLCCheck: a novel serum free light chain assay for the Auxiliary Diagnosis of Multiple Myeloma
- PMID: 40760701
- PMCID: PMC12320346
- DOI: 10.1186/s40001-025-02984-8
Performance and comparison of FLCCheck: a novel serum free light chain assay for the Auxiliary Diagnosis of Multiple Myeloma
Abstract
Background: Multiple myeloma (MM) is a malignant bone marrow disorder characterized by the abnormal proliferation of plasma cells. The measurement of serum free light chains (FLC) is a standard diagnostic and management tool for MM. The International Myeloma Working Group utilizes the iFLC/uFLC ratio to distinguish smoldering multiple myeloma (SMM) from active MM requiring therapeutic intervention.
Objectives: We employed a novel serum free light chain (FLC) assay kit, FLCCheck, to evaluate its consistency with established traditional methods.
Design and methods: We collected 115 samples from 72 patients and tested them using FLCCheck, Freelite, and N Latex FLC. The data obtained from each method were analyzed using Passing-Bablok and Bland-Altman methods, and a chi-square analysis was performed to assess the consistency between the tests. Finally, we used the three methods to calculate and analyze the κ/λ ratio for two patients during their clinical treatment.
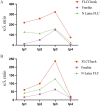
Results: The FLCCheck assay demonstrated a validated linear range, high precision, and strong resistance to interference for both κ and λ light chain detection. By incorporating blocking peptides to reduce cross-reactivity, we improved the reliability of the diluted recovery by more than twofold. The results indicate that FLCCheck exhibits the highest correlation with N Latex FLC, particularly regarding the κ/λ ratio and κ FLC. We also monitored the therapeutic efficacy in MM patients, the results indicated that the three methods followed comparable trends; yet, FLCCheck distinguished itself by demonstrating a more rapid response with the highest amplitude variation.
Conclusions: Our findings show satisfactory consistency between the three FLC testing methods, but their interchangeability is not recommended. Consistent use of the same assay method across all reports is essential. The development of new FLC detection methods is valuable for optimizing disease monitoring, prognosis, and the management of multiple myeloma and related diseases.
Keywords: FLCCheck; Multiple myeloma; Serum free light chain; Turbidimetric immunoassay.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All samples utilized were residual serum obtained following routine clinical laboratory analyses, thus exempting the requirement for informed consent. All procedures were performed in strict adherence to the relevant guidelines and regulations of the local Ethics Committee of the Zhejiang Provincial People’s Hospital on March 5th, 2022 (Approval number: ZJPPHEC 2022SJ (014)). Consent for publication: Not applicable. Competing interest: The authors declare no competing interests.
Figures



References
-
- Caponi L, et al. Inter-assay variability in automated serum free light chain assays and their use in the clinical laboratory. Crit Rev Clin Lab Sci. 2020;57(2):73–85. - PubMed
-
- Dispenzieri A, et al. International myeloma working group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215–24. - PubMed
-
- Napodano C, et al. Mono/polyclonal free light chains as challenging biomarkers for immunological abnormalities. Adv Clin Chem. 2022;108:155–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

