Ancestral Sequence Reconstruction of the Ethylene-Forming Enzyme
- PMID: 40761003
- PMCID: PMC12329707
- DOI: 10.1021/acs.biochem.5c00334
Ancestral Sequence Reconstruction of the Ethylene-Forming Enzyme
Abstract
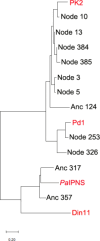
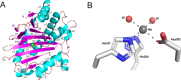
The ethylene-forming enzyme (EFE) catalyzes two main reactions: the conversion of 2-oxoglutarate (2OG) to ethylene plus CO2 and the oxidative decarboxylation of 2OG coupled to the C5 hydroxylation of l-arginine (l-Arg). EFE also facilitates two minor reactions: the uncoupled oxidative decarboxylation of 2OG and the generation of 3-hydroxypropionate (3HP) from 2OG. To better understand the evolution of this enzyme's diverse activities, we demonstrated that two distantly related extant enzymes produce trace levels of ethylene and 3HP, and we examined the reactivities of 11 reconstructed ancestors. The structure of one ancestral protein was resolved by X-ray crystallography, while the others were modeled with AlphaFold2. These studies highlight the importance of residues located at the 2OG and l-Arg binding pockets for the varied activities. For example, effective formation of ethylene requires that the 2OG binding pocket be hydrophobic except for interactions with the substrate carboxylates. Newly identified changes near the l-Arg binding site exhibit significant effects on the reactivities of the enzyme's reactions. Analysis of the reconstructed ancestors suggests that the primordial enzyme exhibited both ethylene-forming and l-Arg hydroxylation activities with partition ratios like the extant examples; i.e., an enzyme capable of catalyzing predominantly one of these reactions did not subsequently develop the ability to affect the secondary reaction.
Figures





References
-
- Fernelius C. W., Wittcoff H., Varnerin R. E.. Ethylene: The organic chemical industry’s most important building block. J. Chem. Educ. 1979;56:385. doi: 10.1021/ed056p385. - DOI
-
- Chenier, P. J. Derivatives of ethylene, In Survey of Industrial Chemistry. Topics in Applied Chemistry, pp 143–162, Springer, Boston, MA, 2002.
-
- Ghanta M., Fahey D., Subramaniam B.. Environmental impacts of ethylene production from diverse feedstocks and energy sources. Appl. Petrochem. Res. 2014;4:167–179. doi: 10.1007/s13203-013-0029-7. - DOI
-
- Gao Y., Neal L., Ding D., Wu W., Baroi C., Gaffney A. M., Li F.. Recent advances in intensified ethylene production--a review. ACS Catal. 2019;9:8592–8621. doi: 10.1021/acscatal.9b02922. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

