Identification of novel molecular subtypes and construction of a prognostic signature via multi-omics analysis and machine learning in lung adenocarcinoma
- PMID: 40761262
- PMCID: PMC12320504
- DOI: 10.3389/fonc.2025.1590216
Identification of novel molecular subtypes and construction of a prognostic signature via multi-omics analysis and machine learning in lung adenocarcinoma
Abstract
Introduction: The development of high-throughput sequencing technologies and targeted therapeutic strategies has significantly improved the prognosis of lung adenocarcinoma (LUAD) patients with sensitive gene mutations. However, patients harboring rare or no actionable mutations were rarely benefit from these targeted therapies. This study aimed to identify novel molecular subtypes and construct a prognostic signature to enhance the stratification of LUAD prognosis.
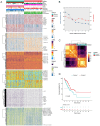
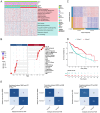
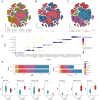
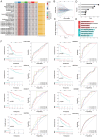
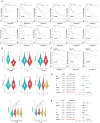
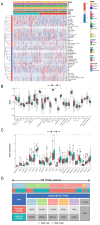
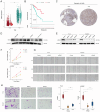
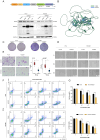
Materials and methods: Novel molecular subtypes of LUAD patients were identified by applying 10 distinct clustering algorithms on multi-omics data. Single-cell RNA-sequencing (scRNA-seq) data were integrated to characterize subtype-specific immune microenvironments. A multi-omics and machine learning-driven prognostic signature (MO-MLPS) was constructed in The Cancer Genome Atlas (TCGA) LUAD dataset using ten machine learning algorithms and subsequently validated across six independent datasets from the Gene Expression Omnibus (GEO) database. The robustness of the model was assessed using the concordance index (C-index), Kaplan-Meier survival analyses, receiver operating characteristic (ROC) curves, and both univariate and multivariate Cox regression analyses. We further confirmed the effects of ANLN knockdown and the expression of a domain-negative anillin protein (dnANLN) via western blotting, cell proliferation assays, flow cytometry, and transwell migration assays in vitro.
Results: Our analysis revealed that the novel molecular subtypes exhibited differences in prognoses, biological functions, and immune infiltration profiles in LUAD. The MO-MLPS was successfully established and validated across TCGA-LUAD cohorts, six independent GEO datasets, and their composite meta-cohort. Higher risk scores from the MO-MLPS correlated with poorer prognosis in LUAD, with AUC values exceeding 0.5 at 1, 3, and 5 years across various cohorts. The signature outperformed 49 previously published prognostic signatures. Furthermore, patients classified as high risk exhibited significantly worse overall and progression-free survival than those classified as low risk. Notably, ANLN knockdown and dnANLN expression significantly inhibited cell proliferation and migration in vitro and enhanced the efficacy of docetaxel.
Conclusion: A comprehensive analysis of multi-omics data redefines the molecular subtype of LUAD patients. The MO-MLPS derived from subtype characteristics has the potential to serve as a clinically valuable prognostic tool. Furthermore, ANLN emerges as a promising novel therapeutic target in the treatment of LUAD.
Keywords: lung adenocarcinoma; machine learning; multi-omics; prognostic signature; single-cell RNA sequencing.
Copyright © 2025 Ma, Xu, Wang, Cao, Yu, Xi, Zhang, Zhan, Liu, Yu, Liu, Liu, Chen and Mai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

