Chemoenzymatic Cascade Synthesis of Metal-Chelating α-Amino Acids
- PMID: 40761323
- PMCID: PMC12320908
- DOI: 10.1002/cctc.202401958
Chemoenzymatic Cascade Synthesis of Metal-Chelating α-Amino Acids
Abstract
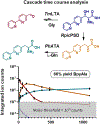
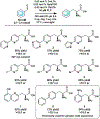
Metal-chelating noncanonical amino acids (ncAAs) are uniquely functional building blocks for proteins, peptide catalysts, and small molecule sensors. However, catalytic asymmetric approaches to synthesizing these molecules are hindered by their functional group variability and intrinsic propensity to ligate metals. In particular, bipyridyl-L-alanine (BpyAla) is a highly sought ncAA, but its complex, inefficient syntheses have limited utility. Here, we develop a chemoenzymatic approach to efficiently construct BpyAla. Three enzymes that can be produced in high titer together react to convert Gly and an aldehyde into the corresponding β-hydroxy ncAA, which is subsequently deoxygenated. We explore approaches to synthesizing biaryl aldehydes and show how the three-enzymatic cascade can access a range of α-amino acids with bulky side chains, including a variety of metal-chelating amino acids. We show that newly accessible BpyAla analogues are compatible with existing amber suppression technology, which will enable future merging of traditional synthetic and biosynthetic approaches to tuning metal reactivity.
Keywords: Biocatalysis; Genetic code expansion; Noncanonical amino acid; Pyridoxal phosphate.
Conflict of interest statement
Conflict of Interests The authors declare no conflict of interest.
Figures
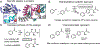
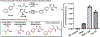

Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Nucleic Acid Nanocapsules as a New Platform to Deliver Therapeutic Nucleic Acids for Gene Regulation.Acc Chem Res. 2025 Jul 1;58(13):1951-1962. doi: 10.1021/acs.accounts.5c00126. Epub 2025 Jun 9. Acc Chem Res. 2025. PMID: 40491030
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Salmonella exploits host- and bacterial-derived β-alanine for replication inside host macrophages.Elife. 2025 Jun 19;13:RP103714. doi: 10.7554/eLife.103714. Elife. 2025. PMID: 40536105 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
-
- Kise KJ, Bowler BE, Tetrahedron Asymmetry 1998, 9, 3319–3324.
Grants and funding
LinkOut - more resources
Full Text Sources
