Chemoenzymatic Cascade Synthesis of Metal-Chelating α-Amino Acids
- PMID: 40761323
- PMCID: PMC12320908
- DOI: 10.1002/cctc.202401958
Chemoenzymatic Cascade Synthesis of Metal-Chelating α-Amino Acids
Abstract
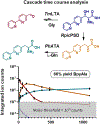
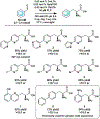
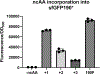
Metal-chelating noncanonical amino acids (ncAAs) are uniquely functional building blocks for proteins, peptide catalysts, and small molecule sensors. However, catalytic asymmetric approaches to synthesizing these molecules are hindered by their functional group variability and intrinsic propensity to ligate metals. In particular, bipyridyl-L-alanine (BpyAla) is a highly sought ncAA, but its complex, inefficient syntheses have limited utility. Here, we develop a chemoenzymatic approach to efficiently construct BpyAla. Three enzymes that can be produced in high titer together react to convert Gly and an aldehyde into the corresponding β-hydroxy ncAA, which is subsequently deoxygenated. We explore approaches to synthesizing biaryl aldehydes and show how the three-enzymatic cascade can access a range of α-amino acids with bulky side chains, including a variety of metal-chelating amino acids. We show that newly accessible BpyAla analogues are compatible with existing amber suppression technology, which will enable future merging of traditional synthetic and biosynthetic approaches to tuning metal reactivity.
Keywords: Biocatalysis; Genetic code expansion; Noncanonical amino acid; Pyridoxal phosphate.
Conflict of interest statement
Conflict of Interests The authors declare no conflict of interest.
Figures
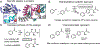
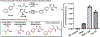

References
-
- Kise KJ, Bowler BE, Tetrahedron Asymmetry 1998, 9, 3319–3324.
Grants and funding
LinkOut - more resources
Full Text Sources
