Astragaloside IV Attenuates Chronic Prostatitis by Activating Keap1/Nrf2/HO-1 Pathway: Suppressing Ferroptosis and Enhancing Antioxidant Defense
- PMID: 40761383
- PMCID: PMC12318839
- DOI: 10.2147/JIR.S527722
Astragaloside IV Attenuates Chronic Prostatitis by Activating Keap1/Nrf2/HO-1 Pathway: Suppressing Ferroptosis and Enhancing Antioxidant Defense
Abstract
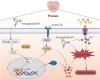
Background: Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), a chronic inflammatory disorder with complex etiology and limited treatment options, is closely associated with oxidative stress and regulates cell death. Ferroptosis-an iron-dependent cell death driven by lipid peroxidation-amplifies CP/CPPS inflammation by concurrently triggering mitochondrial apoptosis and NLRP3 inflammasome activation, while Keap1/Nrf2/HO-1 axis serves as a central regulator bridging ferroptotic, apoptotic, and inflammatory cell death pathways. Astragaloside IV (AS-IV), a primary bioactive component of Astragalus membranaceus with established clinical use in urological therapies and favorable pharmacokinetics, was prioritized over structural analogs due to its unique dual-phase modulation: enhancing Nrf2 nuclear translocation without suppressing NF-κB-mediated immune surveillance. However, regulatory mechanisms linking AS-IV to ferroptosis inhibition in CP/CPPS remain unknown.
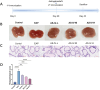
Patients and methods: This study aimed to investigate the therapeutic potential of astragaloside IV (the primary bioactive component of Astragalus membranaceus) for the treatment of CP/CPPS by suppressing ferroptosis via the Keap1/Nrf2/HO-1 pathway. A rat CP/CPPS model was established using complete Freund's adjuvant (CFA), with animals divided into normal control, EAP, and AS-IV high/medium/low-dose groups and treated daily for four weeks. Additionally, a human prostatic epithelial cell (RWPE-1) inflammation model was induced by lipopolysaccharide (LPS), and cells were categorized into control, LPS, AS-IV medium-dose, ferroptosis inhibitor, and Nrf2 inhibitor + AS-IV medium-dose groups.
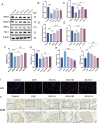
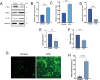
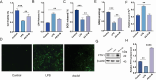
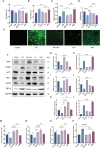
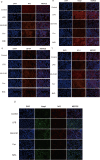
Results: AS-IV ameliorated prostatic tissue inflammation and fibrosis, reduced lipid peroxidation marker malondialdehyde (MDA) levels, and enhanced antioxidant indicators, including glutathione (GSH) content and glutathione peroxidase 4 (GPX4) activity. Western blotting and immunohistochemical analyses further confirmed that AS-IV activated the antioxidant pathway by suppressing Keap1 expression, promoting Nrf2 nuclear translocation, and upregulating heme oxygenase-1 (HO-1) protein levels. Concurrently, pro-inflammatory cytokine levels, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), were markedly reduced.
Conclusion: This is the first study to demonstrate that AS-IV alleviates type III CP pathological damage by inhibiting ferroptosis via the Keap1/Nrf2/HO-1 axis, thereby providing experimental evidence for the development of multi-target therapeutic strategies based on natural products.
Keywords: Nrf2; cellular redox balance; lipid peroxidation; oxidative stress; pharmacological activation; prostatic inflammation.
© 2025 Shi et al.
Conflict of interest statement
The authors have declared that no competing interest exists for this work.
Figures
Similar articles
-
[Ningmitai Capsules alleviate CP/CPPS symptoms by regulating Nrf2 and NF-κB signaling pathways].Zhonghua Nan Ke Xue. 2024 Oct;30(10):889-895. Zhonghua Nan Ke Xue. 2024. PMID: 40783852 Chinese.
-
[Mechanism of Jiming Powder in inhibiting ferroptosis in treatment of myocardial infarction based on NRF2/HO-1/GPX4 pathway].Zhongguo Zhong Yao Za Zhi. 2025 Jun;50(11):3108-3116. doi: 10.19540/j.cnki.cjcmm.20250122.402. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40686179 Chinese.
-
Duhuo jisheng decoction alleviates osteoarthritis progression by mitigating ferroptosis in chondrocytes via the nuclear factor erythroid 2-related factor 2/glutathione peroxidase 4 axis.J Ethnopharmacol. 2025 Aug 17;353(Pt B):120441. doi: 10.1016/j.jep.2025.120441. Online ahead of print. J Ethnopharmacol. 2025. PMID: 40829693
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Ferroptosis and Nrf2 Signaling in Head and Neck Cancer: Resistance Mechanisms and Therapeutic Prospects.Antioxidants (Basel). 2025 Aug 13;14(8):993. doi: 10.3390/antiox14080993. Antioxidants (Basel). 2025. PMID: 40867889 Free PMC article. Review.
References
-
- Ye ZQ, Zeng XY. Advances in the diagnosis and treatment of chronic prostatitis. Natl J Androl. 2003;9(7):483–488. - PubMed
-
- Hua-Wei L, Xiao-Bo D. Prostant combined with α-blockers versus single Prostant for type III prostatitis: a meta-analysis. J Urol. 2017;2017:1.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

