Aerosol inhalation of dimeric artesunate phospholipid-conjugated liposomes ameliorates inflammation, fibrosis, and ferroptosis in neonatal mice with hyperoxia-induced lung injury
- PMID: 40761396
- PMCID: PMC12319227
- DOI: 10.3389/fphar.2025.1542743
Aerosol inhalation of dimeric artesunate phospholipid-conjugated liposomes ameliorates inflammation, fibrosis, and ferroptosis in neonatal mice with hyperoxia-induced lung injury
Abstract
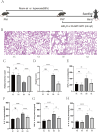
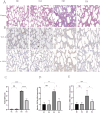
Bronchopulmonary dysplasia (BPD), a chronic lung condition that impacts preterm infants, results in persistent lung damage with limited therapeutic interventions available. Artemisinin, a bioactive compound derived from Artemisia annua, a member of the Asteraceae family, exhibits potent anti-inflammatory and anti-fibrotic characteristics and has been proven to confer protective benefits against acute lung injuries triggered by various factors. However, its potential impact on BPD and the mechanisms involved are not fully understood. This research examines the function and fundamental processes of dimeric artesunate phospholipid-conjugated liposomes (Di-ART-GPC) in BPD. In the in vivo experiments, 48 male neonatal C57BL/6 mice were arbitrarily divided into four cohorts: air (NC cohort), air + Di-ART-GPC (NA cohort), hyperoxia (HO cohort), and hyperoxia + Di-ART-GPC (HA cohort). Mice in the NC and NA cohorts were exposed to normoxic conditions (21% O2) from birth, while those in the HO and HA cohorts were subjected to hyperoxic conditions (95% O2) for 7 days. On the eighth day, NC and NA mice were administered double-distilled water (ddH2O 4 mL), while HO and HA mice received Di-ART-GPC (0.5 mg dissolved in 4 mL ddH2O) via inhalation once daily for 3 days. Lung tissues and serum were harvested on postnatal day 11. Histological evaluations included HE staining for alveolar structure assessment and RAC count and inflammation score quantification; Masson staining for fibrosis evaluation; immunohistochemistry and real-time quantitative PCR (RT-qPCR) for detecting TGF-β1 and α-SMA expression; and ELISA for measuring TNF-α and IL-6 levels. Additional assays quantified superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione (GSH) levels, while immunofluorescence and RT-qPCR assessed Gpx4 expression. For the in vitro component, RAW264.7 macrophages were categorized into the same four cohorts based on culture conditions. Cells in the NC and NA cohorts were cultured under normoxic conditions, while those in the HO and HA cohorts were exposed to 95% O2 for 24 h, following treatment with Di-ART-GPC at 1.25 µM. The supernatant and cells were harvested for subsequent examination. ELISA was employed to measure TNF-α, IL-6, and TGF-β1 levels in the supernatant, while Western blot and RT-qPCR were employed to assess Gpx4 expression in RAW264.7 cells. In vivo findings demonstrated that, in contrast to the NC cohort, the HO cohort exhibited disrupted alveolar architecture, widened alveolar spaces, reduced RAC values, and elevated inflammation and fibrosis scores (p < 0.05). Additionally, the HO cohort demonstrated elevated levels of IL-6 and TNF-α (p < 0.05), higher mRNA expression of TGF-β1 and α-SMA (p < 0.05), reduced SOD activity, diminished GSH content (p < 0.05), and diminished GPX4 protein expression (p < 0.05). Administration of Di-ART-GPC markedly improved these parameters (all p < 0.05). Similarly, in vitro experiments revealed that Di-ART-GPC treatment reduced IL-6, TNF-α, and TGF-β1 levels in hyperoxia-exposed RAW264.7 cells (p < 0.05) and enhanced GPX4 expression (p < 0.05). These findings indicate that Di-ART-GPC demonstrates safeguarding properties against hyperoxia-induced lung damage, potentially by mitigating inflammation and fibrosis in lung tissues and reducing macrophage ferroptosis in hyperoxia-induced BPD.
Keywords: bronchopulmonary dysplasia; ferroptosis; fibrosis; hyperoxia; inflammation; macrophages.
Copyright © 2025 Guan, Chen, Yu, Yu, Chen, Jia, Wang, Cheng and Tian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Bronchopulmonary dysplasia induced by hyperoxia attenuated by A GLP-1 analog, Liraglutide, by regulating the ACE-2/Ang(1-7)/Mas receptor pathway.Pediatr Res. 2025 Sep 2. doi: 10.1038/s41390-025-04293-6. Online ahead of print. Pediatr Res. 2025. PMID: 40897883
-
[Influence and mechanism of extracellular vesicles derived from human dermal papilla cells on skin fibrosis in mice].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2025 Jun 20;41(6):559-568. doi: 10.3760/cma.j.cn501225-20240925-00348. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2025. PMID: 40588404 Free PMC article. Chinese.
-
[Mechanism of Jiming Powder in inhibiting ferroptosis in treatment of myocardial infarction based on NRF2/HO-1/GPX4 pathway].Zhongguo Zhong Yao Za Zhi. 2025 Jun;50(11):3108-3116. doi: 10.19540/j.cnki.cjcmm.20250122.402. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40686179 Chinese.
-
Early (< 8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants.Cochrane Database Syst Rev. 2017 Oct 24;10(10):CD001146. doi: 10.1002/14651858.CD001146.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Oct 21;10:CD001146. doi: 10.1002/14651858.CD001146.pub6. PMID: 29063585 Free PMC article. Updated.
-
Late (≥ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants.Cochrane Database Syst Rev. 2021 Nov 11;11(11):CD001145. doi: 10.1002/14651858.CD001145.pub5. Cochrane Database Syst Rev. 2021. PMID: 34758507 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

