Single-Cell RNA Sequencing Integrated with Bulk-RNA Sequencing Analysis Reveals Prognostic Signatures Based on PANoptosis in Hepatocellular Carcinoma
- PMID: 40761429
- PMCID: PMC12318859
- DOI: 10.2147/JHC.S533777
Single-Cell RNA Sequencing Integrated with Bulk-RNA Sequencing Analysis Reveals Prognostic Signatures Based on PANoptosis in Hepatocellular Carcinoma
Abstract
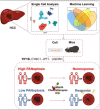
Purpose: Drug resistance severely compromises therapeutic efficacy in hepatocellular carcinoma (HCC); however, the selection of precise treatment strategies for patients remains a critical unmet clinical need. This study investigated PANoptosis-related mechanisms underlying HCC progression to identify actionable therapeutic targets and optimize patient-specific treatment outcomes.
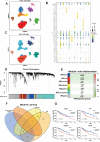
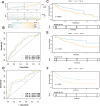
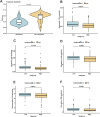
Patients and methods: Multi-omics analysis (single-cell/bulk RNA sequencing) combined with machine learning was used to identify the PANoptosis-related prognostic features. The association of PANoptosis-related expression with the tumor immune microenvironment and drugs was explored using bioinformatic analysis and experimental studies.
Results: High PANoptosis risk exhibited immunosuppressive microenvironments and therapeutic resistance. The PANoptosis-related gene YIF1B has emerged as a dual prognostic biomarker and tumor driver that promotes proliferation, and is linked to immune dysfunction and drug resistance.
Conclusion: YIF1B may be a promising therapeutic target. This PANoptosis framework bridges molecular mechanisms to clinical management, offering strategies for personalized HCC therapy and overcoming treatment resistance.
Keywords: PANoptosis; YIF1B; drug resistant; hepatocellular carcinoma.
© 2025 Wang et al.
Conflict of interest statement
The authors declare no competing interests for this work.
Figures




References
LinkOut - more resources
Full Text Sources

