RNA Epigenetics in Cancer: Current Knowledge and Therapeutic Implications
- PMID: 40761481
- PMCID: PMC12318837
- DOI: 10.1002/mco2.70322
RNA Epigenetics in Cancer: Current Knowledge and Therapeutic Implications
Abstract
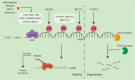
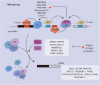
RNA epigenetics, also referred to as epitranscriptomics, has emerged as a critical regulatory layer in cancer biology, extending beyond the scope of traditional DNA and histone modifications. It encompasses a series of dynamic posttranscriptional processes-including RNA biosynthesis, splicing, transport, stability, degradation, translation, and chemical modifications-orchestrated by RNA-binding proteins (RBPs) and noncoding RNAs (ncRNAs). Collectively, these mechanisms influence mRNA fate, shape transcriptional output, and reprogram the tumor microenvironment. Importantly, both coding RNA and ncRNA are themselves subjected to epigenetic regulation, forming intricate feedback loops that contribute to oncogenesis, immune evasion, metastasis, and therapeutic resistance. In this review, we systematically synthesize the current understanding of RNA metabolism and RNA epigenetic modifications during tumor progression, with a particular focus on the roles of RBPs and RNA modifications. Furthermore, we highlight recent advances in RNA-based therapeutic strategies, including mRNA vaccines, antisense oligonucleotides, siRNAs, and circRNA scaffolds. These innovative approaches offer promising avenues for targeting transcriptionally active yet genomically "undruggable" cancer drivers. Together, our synthesis provides a comprehensive framework for understanding RNA epigenetics in tumor biology and lays the groundwork for precision oncology guided by transcriptome plasticity.
Keywords: RNA epigenetics; RNA metabolism; pan‐cancer; targeted therapy.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
RNA modifications in female reproductive physiology and disease: emerging roles and clinical implications.Hum Reprod Update. 2025 Jul 1;31(4):333-360. doi: 10.1093/humupd/dmaf005. Hum Reprod Update. 2025. PMID: 40152541 Review.
-
Chemical Strategies to Modulate and Manipulate RNA Epigenetic Modifications.Acc Chem Res. 2025 Jun 3;58(11):1727-1741. doi: 10.1021/acs.accounts.4c00844. Epub 2025 Mar 18. Acc Chem Res. 2025. PMID: 40100209 Review.
-
Epigenetic alterations in prostate cancer: the role of chromatin remodeling.Epigenomics. 2025 Jul 22:1-25. doi: 10.1080/17501911.2025.2535938. Online ahead of print. Epigenomics. 2025. PMID: 40694614 Review.
-
Hippo/YAP signaling pathway in colorectal cancer: regulatory mechanisms and potential drug exploration.Front Oncol. 2025 Jun 19;15:1545952. doi: 10.3389/fonc.2025.1545952. eCollection 2025. Front Oncol. 2025. PMID: 40612350 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
References
-
- Goodall G. J., Wickramasinghe V. O., “RNA in Cancer,” Nature Reviews Cancer 21, no. 1 (2021): 22–36. - PubMed
-
- Xie R., Chen X., Chen Z., et al., “Polypyrimidine Tract Binding Protein 1 Promotes Lymphatic Metastasis and Proliferation of Bladder Cancer via Alternative Splicing of MEIS2 and PKM,” Cancer Letters 449 (2019): 31–44. - PubMed
-
- Xie R., Cheng L., Huang M., et al., “NAT10 Drives Cisplatin Chemoresistance by Enhancing ac4C‐Associated DNA Repair in Bladder Cancer,” Cancer Research 83, no. 10 (2023): 1666–1683. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
