Bioactive LDH nanoplatforms for cancer therapy: Advances in modulating programmed cell death
- PMID: 40761508
- PMCID: PMC12320707
- DOI: 10.1016/j.mtbio.2025.102139
Bioactive LDH nanoplatforms for cancer therapy: Advances in modulating programmed cell death
Abstract
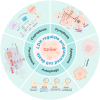
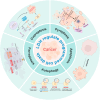
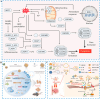
In recent years, the rapid advancement of nanotechnology and tumor biology has significantly expanded the application of nanomaterials in cancer therapy, particularly through the induction of programmed cell death (PCD) in cancer cells. Layered double hydroxides (LDH), a class of two-dimensional inorganic nanomaterials, have attracted considerable attention due to its tunable structures, excellent biocompatibility, and superior drug delivery capabilities. Emerging research has highlighted the great potential of LDH in modulating various forms of PCD. In this review, we provide a comprehensive overview of recent progress in the use of LDH to regulate different PCD pathways in cancer cells, including apoptosis, autophagy, ferroptosis, cuproptosis and pyroptosis. It emphasizes the underlying mechanisms of action, material design strategies, and the application of LDH in precise cancer therapy. Finally, this review is concluded with perspectives on the key challenges and bottlenecks of bioactive LDH in cancer therapy, providing potential solutions and outlining future perspectives.
Keywords: Cancer therapy; Layered double hydroxides; Nanomaterials; Programmed cell death.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA: A Cancer J. Clin. 2022;72(1):7–33. - PubMed
-
- Zheng K., Song R., Li R., Liu M., Ba Y., Jiang W., Fan K. Nanomaterials for refining tumor microenvironment and enhancing therapy in head and neck squamous cell carcinoma: a review. Oncol. Translat. Med. 2024;10(4):151–161.
-
- Qin Y., Zeng W.-F., Liang W. Development of therapeutic cancer vaccines using nanomicellar preparations. Oncol. Translat. Med. 2023;9(6):265–268.
-
- Soerjomataram I., Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020-2070. Nat. Rev. Clin. Oncol. 2021;18(10):663–672. - PubMed
-
- Bai Y., Lam H.C., Lei X. Dissecting programmed cell death with small molecules. Acc. Chem. Res. 2020;53(5):1034–1045. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

