Reconstructing the female reproductive system using 3D bioprinting in tissue engineering
- PMID: 40761511
- PMCID: PMC12320705
- DOI: 10.1016/j.mtbio.2025.102127
Reconstructing the female reproductive system using 3D bioprinting in tissue engineering
Abstract
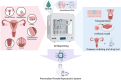
Three-dimensional bioprinting enables the precise fabrication of complex biological tissues through the layer-by-layer deposition of living cells and biomaterials, offering a promising strategy for reconstructing the female reproductive system. This technology has facilitated the development of in vitro models for tissues such as the endometrium, ovary, cervix, and vagina, providing improved structural fidelity and functional relevance. By leveraging bioinks, including decellularized extracellular matrix and advanced bioprinting techniques, researchers can recreate the intricate microarchitectures and vascular networks required for tissue functionality. These bioprinted systems serve as high-fidelity microphysiological systems for studying reproductive health, modeling disease progression, and evaluating therapeutic responses. Moreover, the integration of artificial intelligence into bioprinting workflows enhances reproducibility, scalability, and patient-specific customization. This review summarizes recent advances in reproductive tissue bioprinting and highlights its potential to transform regenerative gynecology and personalized reproductive healthcare.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures










Similar articles
-
3D bioprinting approaches for musculoskeletal interfaces in tissue engineering.Int J Pharm. 2025 Sep 15;682:125939. doi: 10.1016/j.ijpharm.2025.125939. Epub 2025 Jul 6. Int J Pharm. 2025. PMID: 40628344 Review.
-
A bioprinted breast cancer model using bioinks of decellularized breast tissue for studying cancer stemness, invasion, and drug efficacy.Acta Biomater. 2025 Jul 26:S1742-7061(25)00552-5. doi: 10.1016/j.actbio.2025.07.054. Online ahead of print. Acta Biomater. 2025. PMID: 40721188
-
Engineering a microfluidic-assisted 3D bioprinting approach for the hierarchical control deposition and compartmentalisation of graded bioinks.Biofabrication. 2025 Aug 13;17(4). doi: 10.1088/1758-5090/adf35b. Biofabrication. 2025. PMID: 40701169
-
3D bioprinting in tissue engineering: current state-of-the-art and challenges towards system standardization and clinical translation.Biofabrication. 2025 Aug 7;17(4). doi: 10.1088/1758-5090/ade47a. Biofabrication. 2025. PMID: 40513614 Review.
-
Macromolecular crowding-based biofabrication utilizing unmodified extracellular matrix bioinks.Acta Biomater. 2025 May 15;198:37-48. doi: 10.1016/j.actbio.2025.02.052. Epub 2025 Apr 22. Acta Biomater. 2025. PMID: 40268621
References
-
- Deol P.S. Blue Rose Publishers; 2023. Anatomy Physiology of Female Reproductive System.
-
- Bulun S.E. In: Williams Textbook of Endocrinology. thirteenth ed. Melmed S., et al., editors. Elsevier; Philadelphia: 2016. Chapter 17 - physiology and pathology of the female reproductive axis; pp. 589–663.
-
- Critchley H.O.D., et al. Physiology of the endometrium and regulation of menstruation. Physiol. Rev. 2020;100(3):1149–1179. - PubMed
-
- Phillippi J., Kantrowitz-Gordon I. Jones & Bartlett Learning; 2023. Varney's Midwifery.
-
- Rankin J. Elsevier; 2024. Physiology in Childbearing - E-Book: Physiology in Childbearing - E-Book.
Publication types
LinkOut - more resources
Full Text Sources

