Gene Regulatory Changes Associated With Phenological Transitions in an Ecologically Significant Tree Species
- PMID: 40761552
- PMCID: PMC12320121
- DOI: 10.1002/pei3.70078
Gene Regulatory Changes Associated With Phenological Transitions in an Ecologically Significant Tree Species
Abstract
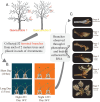
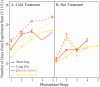
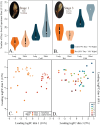
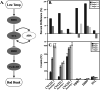
Climate change is driving earlier spring leaf-out across temperate regions, but the genetic mechanisms and environmental interactions underlying this variability are poorly understood. We conducted a controlled growth chamber experiment using excised northern red oak (Quercus rubra ) branches, testing the influence of temperature and photoperiod on leaf development. Two genotypes of red oak were exposed to four different warming and daylength treatments, and gene expression was analyzed across stages of bud development. Results revealed significant phenotypic differences between genotypes and across treatments, confirming that leaf-out timing is both genetically determined and environmentally responsive. Our analysis identified several key genes involved in dormancy break and photoperiod sensitivity, including orthologs to genes identified in Populus species, suggesting conserved pathways across tree species. These genes were differentially expressed in response to environmental factors, highlighting the polygenic nature of phenological timing. Notably, modules associated with temperature and photoperiod showed overlap with dormancy break pathways, indicating shared regulatory networks. This study provides a foundational dataset for understanding phenology in red oak and offers insights into how genetic and environmental factors shape leaf development in temperate trees, setting the stage for further functional genomic research.
Keywords: Quercus rubra; climate change; genotype; growth chamber; phenology; photoperiod; red oak; temperature.
© 2025 The Author(s). Plant‐Environment Interactions published by New Phytologist Foundation and John Wiley & Sons Ltd.
Conflict of interest statement
Benefits generated: This research creates benefits due to all data and results being shared and made available on NCBI SAR.The authors declare no conflicts of interest.
Figures


References
-
- Basler, D. , and Körner C.. 2012. “Photoperiod Sensitivity of Bud Burst in 14 Temperate Forest Tree Species.” Agricultural and Forest Meteorology 165: 73–81.
-
- Blumstein, M. , Oseguera M., Caso‐McHugh T., and Des Marais D. L.. 2024. “Nonstructural Carbohydrate Dynamics' Relationship to Leaf Development Under Varying Environments.” New Phytologist 241, no. 1: 102–113. - PubMed
LinkOut - more resources
Full Text Sources
