Spatial and Temporal Progression of Neurodegeneration in Confirmed and Suspected TDP-43 Type C Pathology
- PMID: 40761602
- PMCID: PMC12320738
- DOI: 10.1162/imag.a.83
Spatial and Temporal Progression of Neurodegeneration in Confirmed and Suspected TDP-43 Type C Pathology
Abstract
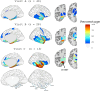
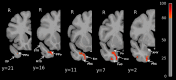
The delineation of disease progression in neurodegenerative entities offers neurobiological insights into pathophysiology as well as practical information on natural progression that can be used to gauge the benefits of treatment. In AD, FTLD-tau and FTLD-TDP types A and B such advances have been achieved based on the investigation of presymptomatic mutation carriers. In the absence of dominant mutations, this approach is not feasible in FTLD-TDP type C (TDP-C). Considering the subtlety of the initial symptoms, it is almost certain that the disease had been progressing for years before the first investigations are obtained. We addressed this limitation through an indirect approach based on the fact that neurodegeneration in TDP-C can be asymmetric for 5-6 years after symptom onset. In time, the contralateral hemisphere starts to show the onset of atrophy, the spread of which mirrors the pattern in the affected hemisphere. The unaffected hemisphere therefore offers an opportunity for capturing the very first emergence of atrophy. To that end, we traced the onset and progression of neurodegeneration in TDP-C by analyzing the right hemisphere longitudinally in cases of asymmetric left anterior temporal atrophy. In these cases, TDP-C was either confirmed at autopsy or suspected based on the clinical features and anatomy of atrophy. Structural MRIs were processed using voxel-based morphometry and parcellated into cortical and subcortical regions. W-scores were computed to identify volume loss relative to age-matched controls. Linear mixed-effects models assessed disease progression across regions of interest (ROIs). Results of our analyses reveal that atrophy in TDP-C follows a stereotyped progression within the right hemisphere, beginning in the ventromedial anterior temporal lobe and extending posteriorly and laterally over time. Early atrophy was most prominent in the medial temporal pole (planum polare), perirhinal cortex, entorhinal cortex, and anterior fusiform cortex, with subcortical involvement initially limited to the amygdala. Voxelwise and ROI-based analyses confirmed that cortical atrophy preceded and exceeded amygdala atrophy in most cases, suggesting greater neocortical vulnerability. Longitudinal linear mixed-effects models identified the greatest volume loss in medial temporal ROIs and the amygdala, following a consistent anterior-to-posterior gradient over time. These findings reconstruct the spatial and temporal progression of TDP-C pathology using the initially unaffected hemisphere as a proxy for early disease stages. The stereotyped trajectory of atrophy aligns with neuropathological patterns and offers critical insights into disease progression, aiding therapeutic evaluation.
Keywords: MRI; Neurodegeneration; TDP-43; atrophy; longitudinal; primary progressive aphasia.
Conflict of interest statement
Competing Interests: The authors declare no competing interests.
Figures


References
-
- Borghesani, V., Battistella, G., Mandelli, M., Welch, A., Weis, E., Younes, K., Neuhaus, J., Grinberg, L., Seeley, W., & Spina, S. (2020). Regional and hemispheric susceptibility of the temporal lobe to FTLD-TDP type C pathology. NeuroImage: Clinical, 28, 102369. 10.1016/j.nicl.2020.102369 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
