Facile synthesis of hydantoin/1,2,4-oxadiazoline spiro-compounds via 1,3-dipolar cycloaddition of nitrile oxides to 5-iminohydantoins
- PMID: 40761763
- PMCID: PMC12320734
- DOI: 10.3762/bjoc.21.118
Facile synthesis of hydantoin/1,2,4-oxadiazoline spiro-compounds via 1,3-dipolar cycloaddition of nitrile oxides to 5-iminohydantoins
Abstract
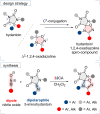
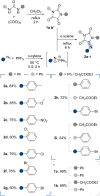
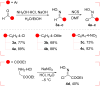
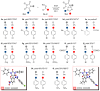
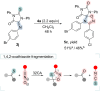
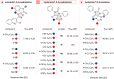
The cycloaddition of 1,3-dipoles at C=N bonds is a relatively rare process, in contrast to the widespread cycloaddition reactions at C=C, C≡C, and C=S bonds. In this study, we present the syntheses of novel hydantoin/1,2,4-oxadiazoline spiro-compounds using a 1,3-dipolar cycloaddition of nitrile oxides to C=N bonds of 5-iminohydantoins. The efficiency of the approach was demonstrated by varying the substituents at four positions of the resulting spirocyclic molecules. Cytotoxicity of the target hydantoin/1,2,4-oxadiazolines was shown to exceed previously known spiro-compounds bearing only hydantoins or 1,2,4-oxadiazolines (IC50 values were 30-50 μM, HCT116 cell lines).
Keywords: 1,3-dipolar cycloaddition; Shiff bases; hydantoins; nitrile oxides; spiro-compounds.
Copyright © 2025, Petrova et al.
Figures
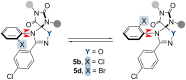
Similar articles
-
Discovery and Chemical Exploration of Spiro[Benzofuran-3,3'-Pyrroles] Derivatives as Innovative FLT3 Inhibitors for Targeting Acute Myeloid Leukemia.Antiinflamm Antiallergy Agents Med Chem. 2025;24(2):127-138. doi: 10.2174/0118715230343474241009112335. Antiinflamm Antiallergy Agents Med Chem. 2025. PMID: 39648426
-
Regioselective Cycloaddition of Nitrile Imines to 5-Methylidene-3-phenyl-hydantoin: Synthesis and DFT Calculations.Int J Mol Sci. 2023 Jan 9;24(2):1289. doi: 10.3390/ijms24021289. Int J Mol Sci. 2023. PMID: 36674803 Free PMC article.
-
[3+2]-Cycloaddition of Nitrile Imines to Parabanic Acid Derivatives-An Approach to Novel Spiroimidazolidinediones.Int J Mol Sci. 2023 Dec 19;25(1):18. doi: 10.3390/ijms25010018. Int J Mol Sci. 2023. PMID: 38203188 Free PMC article.
-
Pulp treatment for extensive decay in primary teeth.Cochrane Database Syst Rev. 2018 May 31;5(5):CD003220. doi: 10.1002/14651858.CD003220.pub3. Cochrane Database Syst Rev. 2018. PMID: 29852056 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
LinkOut - more resources
Full Text Sources
