E2f1 Overexpression Reduces Aging-Associated DNA Damage in Cultured Cerebral Endothelial Cells and Improves Cognitive Performance in Aged Mice
- PMID: 40761766
- PMCID: PMC12321429
- DOI: 10.1155/omcl/3242282
E2f1 Overexpression Reduces Aging-Associated DNA Damage in Cultured Cerebral Endothelial Cells and Improves Cognitive Performance in Aged Mice
Abstract
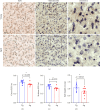
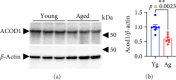
As we age, cerebral endothelial cells (CECs) are less efficient in maintaining genome integrity and accumulate DNA damage. DNA damage in the brain endothelium can lead to the impairment of the blood-brain barrier (BBB), which is a major factor in brain dysfunction and dementia. Thus, identifying factors that regulate DNA repair in the brain endothelium can prevent brain dysfunction associated with aging. E2F1 is a transcription factor that regulates the expression of genes associated with DNA repair, among other functions. We hypothesize that E2F1 is downregulated in the brain vasculature of mice with aging and that E2F1 upregulation can improve cognitive function. We found that in the brain endothelium, E2F1 was significantly less phosphorylated, which is associated with its transcriptional activity, in the brain vasculature of aged mice and cultured CEC derived from aged mice compared with those from young mice. We found that E2f1 overexpression reduced DNA damage in cultured CEC, and targeting the brain vasculature to overexpress E2f1 improved cognition and increased the expression of genes associated with BBB integrity in aged mice. From RNA sequencing data from cultured CEC, we found that E2f1 overexpression significantly upregulated Acod1, which codes for aconitate decarboxylase-1 (ACOD1), an enzyme that produces itaconate. We also found that 4-octyl itaconate (4-OI), a derivative of itaconate, reduced DNA damage, promoted cell proliferation, and restored endothelial barrier function from oxidative stress in cultured CEC. Thus, our study identifies the E2F1-ACOD1 axis as a molecular pathway that can protect the brain endothelium from oxidative stress and aging.
Keywords: DNA damage; E2F1; aging; cognition; endothelial cells; itaconate.
Copyright © 2025 Sheelu Monga et al. Oxidative Medicine and Cellular Longevity published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures







References
-
- López-Otín C., Pietrocola F., Roiz-Valle D., Galluzzi L., Kroemer G. Meta-Hallmarks of Aging and Cancer. Cell Metabolism . 2023;35(1):12–35. - PubMed
-
- Garwood C. J., Simpson J. E., Mashhadi S. Al, et al. DNA Damage Response and Senescence in Endothelial Cells of Human Cerebral Cortex and Relation to Alzheimer’s Neuropathology Progression: A Population-Based Study in the Medical Research Council Cognitive Function and Ageing Study (MRC-CFAS) Cohort. Neuropathology and Applied Neurobiology . 2014;40(7):802–814. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

